|
Prescription drugs have become a significant public health concern, with overtreatment and misuse leading to an alarming number of deaths. The increasing death rate from these drugs is particularly concerning given that many of these fatalities are preventable. The Scale of the ProblemIn 2013, it was estimated that prescription drugs were the third leading cause of death in the United States, following heart disease and cancer. By 2015, it was noted that psychiatric drugs alone also ranked as the third leading cause of death. However, some estimates place prescription drugs as the fourth leading cause, based on a 1998 meta-analysis that primarily considered in-hospital adverse drug reactions. This analysis likely underestimates the true extent of the problem since most drug-related deaths occur outside of hospitals and involve complications that are not always correctly attributed to drug use. Underreported and Misclassified DeathsMany deaths linked to prescription drugs are misclassified as natural or unknown causes. This issue is particularly prevalent with psychiatric drugs, where sudden deaths in young patients are often labeled as natural despite known risks of fatal heart arrhythmias from neuroleptics. Similarly, deaths from depression drugs in the elderly, caused by falls and fractures, often go unrecognized as drug-related. Specific Drug Categories and Risks
Increasing PolypharmacyPolypharmacy, the use of multiple medications by a single patient, has been on the rise, especially among the elderly. This trend increases the risk of adverse drug interactions and fatalities. For example, combining benzodiazepines with neuroleptics significantly raises mortality rates. Estimates of Annual Drug-Related DeathsCurrent estimates suggest that over 882,000 deaths in the United States annually can be attributed to prescription drugs. This figure includes hospital deaths, psychiatric drug fatalities, opioid overdoses, and deaths from NSAIDs. These numbers highlight the magnitude of the problem and the urgent need for intervention. The Role of Misguided Regulation and Lack of AwarenessThe pharmaceutical industry's influence on drug regulation has led to more permissive policies, exacerbating the issue. Many deaths could be prevented if drugs were prescribed more judiciously. For instance, neuroleptics and antidepressants often show minimal efficacy in trials, yet they are widely prescribed. Similarly, NSAIDs are commonly recommended despite their significant risks, often without sufficient consideration of safer alternatives. The pervasive issue of prescription drug-related deaths necessitates a reevaluation of current medical practices and regulatory policies. With most of these deaths being preventable, a more cautious approach to prescribing and better awareness of the risks associated with these medications could save countless lives. It is crucial for healthcare providers, regulators, and patients to acknowledge the dangers and work towards safer, more effective treatment strategies. In bed with big pharma: A $12 Billion RelationshipA comprehensive analysis by Yale University researchers has revealed that nearly six in ten doctors in the United States have received over $12 billion in payments from pharmaceutical and medical device companies over the past decade. This study sheds light on the pervasive financial relationships between healthcare providers and the medical industry, highlighting potential conflicts of interest. Key Findings from the Study
Largest Recipients by SpecialtyOrthopedic surgeons topped the list, receiving the highest total sum of payments at $1.36 billion. They were followed by:
most profitable Drugs and Devices
most prescribed drugsEvery day, millions of people in the U.S. take prescribed drugs in an effort to help them live their lives. As our understanding of medicine has evolved, we’ve developed drugs to aid with some of the most common medical conditions—from pain and blood pressure drugs to asthma medication, thyroid treatments, and antidepressants. This analysis uses prescribed medicines data from the U.S. Agency for Healthcare Research and Quality, released in 2021 for the 2019 calendar year. It also uses supporting drug and health information from MedlinePlus. Top 10 Most Prescribed Drugs in America (2019)
The most prescribed drug, atorvastatin (sold under the brand name Lipitor), was prescribed to 24.5 million people in the U.S. in 2019, or 7.5% of the population. It was one of many statin medications listed, which are claimed to prevent cardiovascular disease and treat abnormal lipid levels. Prevalent Conditions Treated Most of the top prescribed drugs are used to treat high blood pressure or symptoms of it. This is significant as 108 million, or nearly half of adults in the U.S., have hypertension or high blood pressure.
Combining the total patients for blood pressure and cholesterol medications covers 33% of the U.S. population. Pain and inflammation medications were the most frequent on the top 30 list, prescribed to 13.6% of people. Drug Spending in the U.S.A drug’s total number of patients doesn’t necessarily reflect its importance or cost. For example, levothyroxine, the fourth-most prescribed drug by total patients, was the second-most prescribed by total prescriptions with 102.6 million in 2019 at an average cost of $25.10 per prescription. More specialized medications like fluticasone had fewer total prescriptions (27.9 million) but a higher average cost of $97.68 per prescription. Prices are influenced by factors like demand, patent status, and healthcare system variations. Implications and ConcernsThe study underscores ongoing concerns about financial conflicts of interest in the medical field. Researchers noted that such payments might influence physician prescribing behavior and potentially undermine patient trust in medical professionals. Despite these concerns, the practice of accepting industry payments remains widespread. Data Source and MethodologyThe study utilized data from the Open Payments platform, a national database where drug and medical device companies are required to disclose payments made to physicians. This platform aims to increase transparency and help patients make informed decisions about their healthcare providers. FDA recalls and safety concernsIn a related safety concern, the FDA recalled certain Impella devices in December due to a perforation risk that could cause serious injuries or death. This highlights the ongoing need for vigilance regarding the safety of medical devices widely used in clinical practice. ConclusionThis comprehensive analysis by Yale University researchers provides a clear picture of the substantial financial ties between US doctors and the medical industry, emphasizing the need for ongoing scrutiny and transparency. The relationship between healthcare providers and the medical industry is complex and often financially intertwined. While these financial interactions can support medical education and innovation, they also pose significant ethical and practical challenges. Ensuring transparency and addressing potential conflicts of interest are crucial steps toward maintaining the integrity of medical practice and patient trust. referencesGøtzsche PC. Deadly Medicines and Organised Crime: How Big Pharma Has Corrupted Health Care. London: Radcliffe Publishing; 2013.
Gøtzsche PC. Deadly Psychiatry and Organised Denial. Copenhagen: People’s Press; 2015. Schroeder MO. Death by Prescription: By one estimate, taking prescribed medications is the fourth leading cause of death among Americans. US News 2016; Sept 27. Light DW, Lexchin J, Darrow JJ. Institutional corruption of pharmaceuticals and the myth of safe and effective drugs. J Law Med Ethics 2013;41:590-600. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998;279:1200–5. FAERS Reporting by Patient Outcomes by Year. FDA 2015;Nov 10. Gøtzsche PC. Mental Health Survival Kit and Withdrawal From Psychiatric Drugs. Ann Arbor: L H Press; 2022. Hubbard R, Farrington P, Smith C, et al. Exposure to tricyclic and selective serotonin reuptake inhibitor antidepressants and the risk of hip fracture. Am J Epidemiol 2003;158:77-84. Thapa PB, Gideon P, Cost TW, et al. Antidepressants and the risk of falls among nursing home residents. N Engl J Med 1998;339:875-82. Ebbesen J, Buajordet I, Erikssen J, et al. Drug-related deaths in a department of internal medicine. Arch Intern Med 2001;161:2317–23. James JTA. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf 2013;9:122-8. Ho JY. Life Course Patterns of Prescription Drug Use in the United States. Demography 2023;60:1549-79. Gøtzsche PC. Long-term use of antipsychotics and antidepressants is not evidence-based. Int J Risk Saf Med 2020;31:37-42. Gøtzsche PC. Long-Term Use of Benzodiazepines, Stimulants and Lithium is Not Evidence-Based. Clin Neuropsychiatry 2020;17:281-3. Forbruget af antipsykotika blandt 18-64 årige patienter, med skizofreni, mani eller bipolar affektiv sindslidelse. København: Sundhedsstyrelsen; 2006. Hughes S, Cohen D, Jaggi R. Differences in reporting serious adverse events in industry sponsored clinical trial registries and journal articles on antidepressant and antipsychotic drugs: a cross-sectional study. BMJ Open 2014;4:e005535. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005;294:1934–43. FDA package insert for Risperdal (risperidone). Accessed 30 May 2022. Koponen M, Taipale H, Lavikainen P, et al. Risk of Mortality Associated with Antipsychotic Monotherapy and Polypharmacy Among Community-Dwelling Persons with Alzheimer’s Disease. J Alzheimers Dis 2017;56:107-18. Whitaker R. Lure of Riches Fuels Testing. Boston Globe 1998; Nov 17. Whitaker R. Mad in America: Bad science, Bad medicine, and the Enduring Mistreatment of the Mentally Ill. Cambridge: Perseus Books Group; 2002:page 269. Vanderburg DG, Batzar E, Fogel I, et al. A pooled analysis of suicidality in double-blind, placebo-controlled studies of sertraline in adults. J Clin Psychiatry 2009;70:674-83. Hengartner MP, Plöderl M. Newer-Generation Antidepressants and Suicide Risk in Randomized Controlled Trials: a Re-Analysis of the FDA Database. Psychother Psychosom 2019;88:247-8. Hengartner MP, Plöderl M. Reply to the Letter to the Editor: “Newer-Generation Antidepressants and Suicide Risk: Thoughts on Hengartner and Plöderl’s ReAnalysis.” Psychother Psychosom 2019;88:373-4. Weich S, Pearce HL, Croft P, et al. Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: retrospective cohort study. BMJ 2014;348:g1996. Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: a matched cohort study. BMJ Open 2012;2:e000850. Coupland C, Dhiman P, Morriss R, et al. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ 2011;343:d4551. Smoller JW, Allison M, Cochrane BB, et al. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women’s Health Initiative study. Arch Intern Med 2009;169:2128-39. O’Neill A. Age distribution in the United States from 2012 to 2022. Statista 2024;Jan 25. Olfson M, King M, Schoenbaum M. Antipsychotic Treatment of Adults in the United States. Psychiatrist.com 2015;Oct 21. Maust DT, Lin LA, Blow FC. Benzodiazepine Use and Misuse Among Adults in the United States. Psychiatr Serv 2019;70:97-106. Brody DJ, Gu Q. Antidepressant Use Among Adults: United States, 2015-2018. CDC 2020; Sept. Centers for Disease Control and Prevention. Leading Causes of Death. 2024; Jan 17. Drug Overdose Deaths. Centers for Disease Control and Prevention 2023; Aug 22. Davis JS, Lee HY, Kim J, et al. Use of non-steroidal anti-inflammatory drugs in US adults: changes over time and by demographic. Open Heart 2017;4:e000550. Conaghan PG. A turbulent decade for NSAIDs: update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol Int 2012;32:1491-502. Bally M, Dendukuri N, Rich B, et al. Risk of acute myocardial infarction with NSAIDs in real world use: bayesian meta-analysis of individual patient data. BMJ 2017;357:j1909. Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular Events Associated with Rofecoxib in a Colorectal Adenoma Chemoprevention Trial. N Engl J Med 2005;352:1092-102. Blower AL, Brooks A, Fenn GC, et al. Emergency admissions for upper gastrointestinal disease and their relation to NSAID use. Aliment Pharmacol Ther 1997;11:283–91. Davis C, Lexchin J, Jefferson T, Gøtzsche P, McKee M. “Adaptive pathways” to drug authorisation: adapting to industry? BMJ 2016;354:i4437. van der Hooft CS, Sturkenboom MC, van Grootheest K, et al. Adverse drug reaction-related hospitalisations: a nationwide study in The Netherlands. Drug Saf 2006;29:161-8. Gøtzsche PC. Big marketing hoax: Non-steroidal, anti-inflammatory drugs (NSAIDs) are not anti-inflammatory. Copenhagen: Institute for Scientific Freedom 2022;Nov 10. Perlis R. The time has come for over-the-counter antidepressants. Stat News 2024;April 8. Gøtzsche PC. Critical Psychiatry Textbook. Copenhagen: Institute for Scientific Freedom; 2022. Freely available. Tilley, Caitlin. “Corruption Fears as Report Finds US Doctors Received Record $12bn.” Mail Online, 3 Apr. 2024, www.dailymail.co.uk/health/article-13268371/Corruption-doctors-received-pharma-payments.html. Tanne, Janice Hopkins. “US Doctors Received More than $12bn in Industry Payments between 2013 and 2022, Study Shows.” BMJ, vol. 385, 2 Apr. 2024, p. q781, www.bmj.com/content/385/bmj.q781.full, https://doi.org/10.1136/bmj.q781.
0 Comments
Since the declaration of the COVID-19 pandemic by the World Health Organization (WHO) on March 11, 2020, over 13.5 billion doses of COVID-19 vaccines have been administered worldwide. This remarkable achievement in vaccine distribution highlights the urgent need for comprehensive vaccine safety monitoring, as very rare adverse events associated with COVID-19 vaccines may only become apparent after widespread administration. To address this need, the Safety Platform for Emergency Vaccines (SPEAC) initiative formulated a list of potential COVID-19 vaccine adverse events of special interest (AESI) in 2020. These AESI were selected based on various factors, including their associations with immunization, vaccine platforms, or adjuvants, as well as theoretical concerns related to immunopathogenesis. One flexible approach for assessing AESI is the comparison of observed AESI rates following vaccine introduction with expected rates based on historical periods pre-vaccine rollout. This method, known as observed vs. expected (OE) analysis, can rapidly detect potential vaccine safety signals. For example, OE analysis played a crucial role in identifying thrombosis with thrombocytopenia syndrome (TTS) as a safety signal, prompting the suspension of the AstraZeneca COVID-19 vaccine in certain countries. To further enhance vaccine safety monitoring, a global cohort study was conducted as part of the Global COVID Vaccine Safety (GCoVS) Project. This project, funded by the Centers for Disease Control and Prevention (CDC), involves multiple nations and aims to monitor COVID-19 vaccine safety on a global scale. Thirteen AESI were selected for evaluation, including neurological, hematologic, and cardiovascular conditions, which are as follows:
The study analyzed data from 10 sites across eight countries, comprising a total vaccinated population of 99,068,901 individuals. Notable findings include a statistically significant increase in Guillain-Barré syndrome (GBS) cases following the administration of the ChAdOx1 (India) vaccine and an increased risk of acute disseminated encephalomyelitis (ADEM) after the mRNA-1273 vaccine (Moderna). Hematologic conditions such as cerebral venous sinus thrombosis (CVST) and immune thrombocytopenia (ITP) also showed elevated risk ratios following certain vaccine doses. Similarly, cardiovascular conditions like myocarditis and pericarditis demonstrated increased risk ratios, particularly after mRNA vaccine doses (Pfizer, Moderna, AstraZeneca). Here is the raw data collected from the study: Here is a chart summarizing the raw data collected in the study: Overall, these findings underscore the importance of ongoing vaccine safety monitoring and highlight the value of global collaboration in assessing vaccine-related adverse events. By leveraging methodologies such as OE analysis and conducting comprehensive cohort studies, public health agencies can swiftly detect and respond to potential vaccine safety signals, ensuring the continued safety and effectiveness of COVID-19 vaccination efforts worldwide. referencesK. Faksova, et al. “COVID-19 Vaccines and Adverse Events of Special Interest: A Multinational Global Vaccine Data Network (GVDN) Cohort Study of 99 Million Vaccinated Individuals.” Vaccine, 1 Feb. 2024, https://doi.org/10.1016/j.vaccine.2024.01.100.
The human gut is teeming with a diverse array of bacteria collectively known as the gut microbiota. Among its many functions, one of the most vital is colonization resistance—the ability to prevent harmful pathogens from taking up residence in the gut and causing disease. However, understanding which microbiota communities are protective and which allow pathogens to thrive has long been a challenge. In a groundbreaking study led by Spragge et al., researchers shed light on the complex dynamics of gut microbiota and their role in colonization resistance against two significant bacterial pathogens: Klebsiella pneumoniae and Salmonella enterica serovar Typhimurium. Their findings, published in Science, unveil the critical importance of microbiome diversity in safeguarding against pathogenic invasion. Traditionally, it was believed that certain individual bacterial species might confer colonization resistance. However, Spragge et al. discovered that the true protective power lies in the collective diversity of the microbiota. They conducted meticulous experiments both in vitro and in gnotobiotic mice (mice that have been raised in a controlled environment where the microbial composition of their gut is precisely known and controlled), evaluating the ability of single bacterial species and increasingly diverse microbiota communities to resist pathogen colonization. Surprisingly, the researchers found that single species alone provided limited protection against the pathogens. It was only when these species were combined into diverse communities consisting of up to 50 different species that colonization resistance was significantly enhanced. This underscores the importance of ecological diversity in promoting gut health. Moreover, the study identified certain key species within these diverse communities that played a pivotal role in bolstering colonization resistance, even though they offered little protection on their own. These key species acted by consuming nutrients required by the pathogens, thereby depriving them of essential resources for growth and establishment in the host. Importantly, Spragge et al. demonstrated that microbiome diversity not only increases the probability of protection against pathogens but also enhances the overlap in nutrient utilization profiles between the microbiota community and the pathogen. This nutrient blocking mechanism serves as a potent defense strategy against pathogenic invasion. The implications of these findings are profound. They provide compelling evidence for the health benefits of a diverse gut microbiome and offer insights into the rational design of pathogen-resistant microbiota communities. By harnessing the protective power of microbiome diversity, we may pave the way for innovative strategies to combat infectious diseases and promote overall gut health. In conclusion, Spragge et al.'s study unveils the intricate interplay between microbiome diversity and colonization resistance, highlighting the collective strength of diverse bacterial communities in defending against pathogenic threats. This research not only expands our understanding of gut microbiota dynamics but also holds promise for the development of novel therapeutics aimed at fortifying the body's natural defenses against infections. referencesSpragge, Frances, et al. “Microbiome Diversity Protects against Pathogens by Nutrient Blocking.” Science, vol. 382, no. 6676, 15 Dec. 2023, https://doi.org/10.1126/science.adj3502.
In recent years, researchers have uncovered a surprising trend in human physiology: a decline in body temperature over the past two centuries. Contrary to the long-standing belief that the normal body temperature is 37°C (98.6°F), studies spanning multiple cohorts and time periods have revealed a consistent decrease in average body temperature, suggesting a real physiological change rather than a mere artifact of measurement bias. A groundbreaking study analyzed data from three cohorts spanning 157 years (over 600,000 data inputs), including Union Army Veterans of the Civil War, the National Health and Nutrition Examination Survey I, and the Stanford Translational Research Integrated Database Environment. The findings revealed a monotonic decrease in body temperature, with men born in the early 19th century having temperatures 0.59°C higher than men today. A similar decline was observed in women, indicating a significant shift in human physiology over time. While some may attribute these findings to changes in measurement methods or biases, the study's authors argue that the observed drop in temperature reflects real physiological differences. Human body temperature is a crude surrogate for basal metabolic rate. These findings of a decrease in body temperature indicate a decrease in metabolic rate, which is supported in the literature when comparing modern experimental data to those from 1919. Resting metabolic rate, a key component of daily energy expenditure, has been linked to body temperature and longevity. The observed decrease in body temperature suggests a corresponding decline in metabolic rate, independent of changes in body size. This likely has implications for human health and longevity, as metabolic health underlies all vital organ functions. Additionally, changes in ambient temperature and the widespread adoption of heating and cooling systems in modern times may have influenced body temperature trends. Increased time spent in thermoneutral zones, where minimal energy is expended to maintain body temperature, could contribute to the observed decline in resting metabolic rate and body temperature. In conclusion, body temperature has declined, implying lower metabolic rates (since heat is generated as a byproduct of energy production). This declining trend in human body temperature over the past two centuries offers valuable insights into the complex interplay between physiology, environment, and health. As researchers continue to unravel the mysteries of human biology, these findings pave the way for a deeper understanding of our species' evolution and resilience in the face of changing environments and lifestyles. referencesProtsiv, Myroslava, et al. “Decreasing Human Body Temperature in the United States since the Industrial Revolution.” ELife, vol. 9, 7 Jan. 2020, p. e49555, elifesciences.org/articles/49555, https://doi.org/10.7554/eLife.49555.
Dai, Dao-Fu, et al. “Mitochondrial Oxidative Stress in Aging and Healthspan.” Longevity & Healthspan, vol. 3, no. 1, 2014, p. 6, https://doi.org/10.1186/2046-2395-3-6. In our modern world, where convenience often comes at a cost, the prevalence of obesogens – chemicals that disrupt the body's normal metabolism and contribute to weight gain – has emerged as a growing concern. From everyday products to industrial pollutants, obesogens permeate our environment, exerting subtle yet profound effects on our health and well-being. Commonly encountered obesogensAmong the many obesogens encountered in daily life, several stand out for their widespread use and potential health impacts:
Mechanisms of ActionObesogens exert their effects through various mechanisms, including:
Disruption of Metabolism via MitochondriaObesogens, through their pervasive presence in our environment, exert insidious effects on metabolic function, including the intricate workings of mitochondria – the cellular powerhouses responsible for energy production. By disrupting mitochondrial function, obesogens can contribute to metabolic dysregulation and, ultimately, weight gain. Mitochondria play a central role in energy metabolism, converting nutrients into adenosine triphosphate (ATP), the primary source of cellular energy. However, exposure to obesogens can impair mitochondrial function through various mechanisms, including:
The disruption of mitochondrial function by obesogens can have profound implications for metabolic health and contribute to obesity through several pathways:
causative relationship with health conditionsThe impact of obesogens on human health extends beyond weight gain, with associations documented with various health conditions, including:
Additionally, obesogens are highly related to the following health conditions and physiologic imbalances:
Unraveling the Role of Dysfunctional Adipose TissueRelatively little is known about the extent to which obesogen exposure programs dysfunctional adipose tissue that may store but not mobilize fat. However, emerging evidence suggests that obesogens may contribute to adipocyte dysfunction, leading to altered fat storage and metabolism. One potential underlying factor is suboptimal liver detoxification pathways due to inadequate micronutrient cofactors. Inadequate levels of essential micronutrients, such as vitamins and minerals, can impair liver detoxification pathways responsible for metabolizing and eliminating obesogens from the body. As a result, obesogens may accumulate in adipose tissue, disrupting metabolic function and contributing to weight gain. Additionally, micronutrient deficiencies can compromise mitochondrial function, further exacerbating metabolic dysfunction and obesity risk. A Layman's Overview of Obesogens: Redefining the Weight Loss ParadigmIn the quest for weight loss, many of us often find ourselves fixating on calorie counting, fad diets, or intense workout regimens. However, what if I told you that the key to achieving a healthy weight isn't solely about shedding pounds but rather fixing your metabolism? Enter obesogens – a lesser-known yet influential factor in the obesity epidemic. As mentioned, obesogens are chemicals found in our environment, ranging from pesticides and plastics to food additives and personal care products. These substances have the uncanny ability to disrupt our body's natural weight-regulating mechanisms, leading to weight gain and metabolic dysfunction. Instead of solely blaming calories in versus calories out, it's essential to recognize the role obesogens play in shaping our metabolism. The Better Question: Fixing MetabolismRather than constantly asking ourselves, "How do I lose weight?" a more pertinent question would be: "How do I fix my metabolism?" Fixing metabolism involves addressing the root cause of weight gain – obesogen exposure and metabolic disruption. By eliminating or reducing our exposure to obesogens and ensuring our bodies receive essential micronutrients, we can optimize metabolic function and promote overall health. The Two-Fold SolutionTo achieve optimal health and maintain a healthy weight, a two-fold approach is necessary: 1. Reduce Toxin Exposure: Minimize exposure to obesogens by making conscious choices in our daily lives. This includes opting for organic produce, using natural cleaning and personal care products, and avoiding plastic containers and food packaging whenever possible. By participating in a structured evidenced-based detoxification program, we in turn lower our toxic burden, and we can mitigate the adverse effects of obesogens on our metabolism. 2. Consume Micronutrients: Vital micronutrients, such as vitamins and minerals, serve as essential cofactors in metabolic pathways. Ensuring adequate intake of these micronutrients through a balanced diet rich in fruits, vegetables, whole grains, and lean proteins can support optimal metabolic function. Additionally, supplementation may be necessary to address any deficiencies and promote metabolic health. The conventional approach to weight loss often overlooks the critical role obesogens play in metabolic dysfunction. Instead of solely focusing on calorie restriction or intense exercise, shifting our focus to fixing metabolism through toxin reduction and micronutrient consumption offers a more holistic and sustainable solution to achieving optimal health. By addressing the underlying factors contributing to metabolic disruption, we can pave the way for lasting weight management and overall well-being. the harm of environmental toxinsThe disruption of metabolic and mitochondrial function by obesogens represents a significant public health concern, with implications for obesity and metabolic disease. By understanding the mechanisms through which obesogens impair mitochondrial function and contribute to weight gain, researchers can develop targeted interventions to mitigate their adverse effects on metabolic health. Moreover, addressing underlying factors such as suboptimal liver detoxification pathways and micronutrient deficiencies is essential in combating the detrimental impact of obesogens on metabolic function and obesity prevalence. The pervasive presence of obesogens in our environment underscores the need for greater awareness and regulation of these harmful chemicals. By minimizing exposure to obesogens and advocating for safer alternatives, we can mitigate their adverse effects on human health and combat the rising tide of obesity and metabolic disease. As we navigate the complexities of modern living, vigilance and informed consumer choices are essential in safeguarding our health and well-being against the hidden threats of obesogens. Taking Action: The Integral Wellness ProgramFor those seeking tangible solutions to combat the effects of obesogens and improve their overall well-being, the Integral Wellness Program offers a comprehensive approach to optimizing health and vitality. This flagship service provides personalized guidance and support in key areas of movement, nutrition, and lifestyle to directly enhance quality of life. Online/In-Person Guidance One of the standout features of the Integral Wellness Program is its flexibility, offering both online and in-person consultations tailored to individual preferences and needs. Whether you prefer the convenience of virtual sessions or the hands-on approach of in-person coaching, our team of experienced wellness professionals is dedicated to supporting you every step of the way. Movement, Nutrition, and Lifestyle The Integral Wellness Program takes a holistic approach to health, addressing modifiable factors and behaviors in three core areas:
Augmenting the Health Process By participating in the Integral Wellness Program, you'll not only gain valuable knowledge and skills to navigate the challenges of modern living but also receive ongoing support and accountability to stay on track towards your health goals. Through targeted interventions aimed at eliminating obesogen exposure and promoting healthy behaviors, you can unlock your body's full potential and thrive in all aspects of life. The Integral Wellness Program offers a transformative journey towards optimal health and vitality. By prioritizing movement, nutrition, and lifestyle modifications, participants can take proactive steps to combat the effects of obesogens and reclaim control over their well-being. With the guidance and support of our dedicated wellness professionals, you'll embark on a path of self-discovery, empowerment, and lasting transformation. referencesThis article challenges the conventional understanding of heart disease, particularly the widely accepted theory that attributes its cause primarily to events occurring in the coronary arteries. Instead, a paradigm shift is proposed, contending that a deeper understanding of heart disease, encompassing angina, unstable angina, and myocardial infarction (heart attack), necessitates a focus on events within the myocardium, the muscular tissue of the heart. Over the past decades, the prevailing belief in the coronary artery theory has led to costly surgical interventions, widespread medication use with questionable benefits, and dietary recommendations that may exacerbate rather than alleviate the problem. By delving into the precise pathophysiological events that underlie heart attacks, we can uncover alternative approaches to prevention and treatment, such as adopting a "Nourishing Traditions"-style diet and utilizing safe and affordable medicines like g-strophanthin. Furthermore, this shift in perspective prompts us to confront broader issues, including the impact of modern lifestyles on human health, the need for a new medical paradigm, and the importance of ecological consciousness. Ultimately, reexamining the root causes of heart disease offers a pathway to addressing this pervasive health challenge and forging a healthier future for all. The information is summarized based on the work of Dr. Thomas Cowan, vice president of the Physicians Association for Anthroposophical Medicine and is a founding board member of the Weston A. Price Foundation. During his career he has studied and written about many subjects in medicine. These include nutrition, homeopathy, anthroposophical medicine, and herbal medicine. Challenging the Conventional model: Revisiting the Causes of Heart AttacksThe traditional understanding of heart attacks, largely centered on arterial blockage due to plaque buildup, has faced challenges in recent years. Initially, it was believed that blockages in the major coronary arteries led to oxygen deficiency in the heart, causing chest pain (angina) and eventually progressing to a heart attack. This simplistic view prompted invasive procedures like angioplasty, stents, and coronary bypass surgery as standard treatments. However, clinical observations and research findings have cast doubts on this approach. Anecdotal evidence (admittedly low quality evidence) from a trial in rural Alabama revealed surprising outcomes among individuals with single artery blockages. Contrary to expectations, less than 10% of those who experienced heart attacks did so in the region of the heart supplied by the blocked artery. Similarly, a comprehensive study conducted by the Mayo Clinic highlighted the limited efficacy of bypass surgery in preventing future heart attacks. While the procedure offered relief from chest pain, it did not significantly reduce the risk of subsequent heart events, except in high-risk patients. Contrary to popular belief, blockages exceeding 90% are often compensated for by collateral blood vessels, which develop over time to ensure uninterrupted blood flow to the heart. This extensive network of collateral vessels serves as a natural bypass system, mitigating the impact of arterial blockages on blood circulation. However, diagnostic procedures like coronary angiograms, which rely on injecting heavy dye into the arteries, often fail to accurately assess the extent of blockages and the true blood flow in the heart. As a result, many patients undergo invasive treatments such as bypass surgery, stents, or angioplasty based on misleading information about the severity of their arterial blockages. Moreover, studies have shown that these procedures provide minimal benefit, if any, to patients, particularly those with minimally symptomatic blockages exceeding 90%. Despite the widespread use of these interventions, their efficacy in restoring blood flow and preventing heart attacks remains questionable. These revelations underscore the need for a reevaluation of conventional treatment strategies and a deeper exploration of the underlying mechanisms behind heart attacks. Rather than focusing solely on arterial blockages, a more holistic approach that considers factors beyond plaque buildup may offer greater insights into the prevention and management of heart disease. Beyond the Coronary Artery TheoryThe prevailing focus in cardiology has long been on the stable, progressing plaque within the coronary arteries, deemed responsible for heart attacks. However, recent insights challenge this notion, redirecting attention to the unpredictable nature of unstable plaques. Unlike their calcified counterparts, unstable plaques are soft and prone to rapid evolution, abruptly occluding arteries and triggering downstream oxygen deficits, angina, and ischemia. These vulnerable plaques are believed to be a blend of inflammatory buildup and low-density lipoprotein (LDL), the primary targets of statin drugs. Consequently, the widespread adoption of statin therapy is advocated as a preventive measure against heart attacks, fueled by angiogram studies purportedly showcasing the prevalence of unstable plaques as the leading cause of myocardial infarctions (MIs). Yet, autopsies and pathology studies present a different narrative. Thrombosis, deemed crucial in precipitating MIs, is found in only a fraction of cases upon meticulous examination. Furthermore, measurements of myocardial oxygen levels during MIs reveal no discernible deficit, challenging the conventional understanding of ischemia as the primary mechanism. While thrombosis does occur in conjunction with MIs, its occurrence in less than half of cases underscores the inadequacy of attributing MIs solely to arterial blockages. The timing of thrombosis, often post-MI, begs the question: what precipitated the event in the first place? These inconsistencies underscore the limitations of existing theories surrounding coronary artery involvement in MIs. As the spotlight shifts away from stable plaques, a pressing question emerges: What truly underlies the genesis of heart attacks? Unveiling the Autonomic Symphony: The Heart's Harmonious BalanceAn accurate understanding of myocardial ischemia necessitates consideration of the primary risk factors associated with heart disease, including gender, diabetes, smoking, and chronic psychological stress. Curiously, none of these risk factors directly implicate coronary artery pathology; instead, they impact capillary health or exert indirect effects. Over the past five decades, key medications in cardiology, such as beta-blockers, nitrates, aspirin, and statins, have demonstrated some benefits for heart patients. However, their mechanisms of action must be scrutinized within a comprehensive theory of myocardial ischemia. A groundbreaking revelation in heart disease prevention and treatment stems from the autonomic nervous system's role in ischemia genesis, as illuminated by heart-rate variability monitoring. The autonomic nervous system comprises two branches—the sympathetic and parasympathetic—responsible for regulating physiological responses. Imbalance between these branches emerges as a significant contributor to heart disease. Studies reveal a notable reduction in parasympathetic activity among patients with ischemic heart disease, particularly preceding ischemic events triggered by physical or emotional stressors. Conversely, abrupt increases in sympathetic activity rarely culminate in ischemia without antecedent parasympathetic decline. Notably, women exhibit stronger vagal activity than men, potentially influencing sex-based disparities in MI incidence. Multiple risk factors, including hypertension, smoking, diabetes, and stress, diminish parasympathetic activity, underscoring the pivotal role of the regenerative nervous system in heart health. Conversely, pharmacological interventions like nitrates, aspirin, and statins stimulate parasympathetic mediators, promoting ANS balance. In essence, while traditional risk factors and interventions influence plaque and stenosis development, their paramount impact lies in restoring ANS equilibrium. Thus, understanding the sequence of events leading to myocardial infarction demands a deeper exploration of autonomic nervous system dynamics. The Underlying pathophysiology of Myocardial IschemiaIn the vast majority of cases, the pathology leading to myocardial infarction (MI) begins with a decreased tonic activity of the parasympathetic nervous system (rest and digest), often exacerbated by physical or emotional stressors. This reduction prompts an increase in sympathetic nervous system activity, triggering heightened adrenaline production and directing myocardial cells to break down glucose via aerobic glycolysis, rather than their preferred fuel source of ketones and fatty acids (often explaining why patients report feeling tired before a MI). Remarkably, despite these metabolic shifts, no change in blood flow, as measured by the myocardial cell oxygen level (pO2), occurs. The shift towards glycolysis results in a surge of lactic acid production within myocardial cells, a phenomenon observed in nearly all MIs. This surge, coupled with localized tissue acidosis, impedes calcium entry into cells, compromising their contractility. Consequently, localized edema ensues, leading to hypokinesis—the hallmark of ischemic disease—and eventual tissue necrosis characteristic of an MI. Moreover, the ensuing tissue edema alters arterial hemodynamics, escalating sheer pressure and exacerbating plaque instability. This process elucidates the rupture of unstable plaques and their role in exacerbating arterial blockage during critical, acute scenarios. This explanation accounts for all the observable phenomena associated with heart disease. Understanding the etiology of heart disease holds profound implications beyond academic curiosity. It informs therapeutic strategies aimed at preserving parasympathetic activity, fostering holistic approaches to heart health, and challenging prevailing "civilized" industrial lifestyles. Central to this paradigm shift is the recognition of the vital role played by g-strophanthin—a hormone derived from the strophanthus plant. G-strophanthin is an endogenous hormone made in the adrenal cortex from cholesterol, whose production is inhibited by statin drugs, that does two things that are crucial for heart health and are done by no other medicine. G-strophanthin uniquely stimulates the production of acetylcholine, the primary neurotransmitter of the parasympathetic nervous system, while also converting lactic acid—the metabolic poison implicated in ischemic processes—into pyruvate, a preferred myocardial cell fuel. Perhaps this “magic” is why Chinese medicine practitioners say that the kidneys (i.e., adrenals, where ouabain is made) nourish the heart. Embracing this understanding not only guides therapeutic interventions but also underscores the imperative of dietary modifications. A diet abundant in healthful fats and fat-soluble nutrients, while low in processed carbohydrates and sugars, emerges as a cornerstone of heart health—a departure from the industrialized diets synonymous with modern civilization. In essence, unraveling the metabolic symphony orchestrating myocardial ischemia offers a transformative lens through which to perceive heart disease, fostering a holistic approach that transcends conventional paradigms and embraces the profound interconnectedness of mind, body, and environment. referencesGiorgio Baroldi. The Etiopathogenesis of Coronary Heart Disease. CRC Press EBooks, Informa, 20 Jan. 2004. Accessed 29 Mar. 2024.
Sroka K. On the genesis of myocardial ischemia. Z Kardiol. 2004 Oct;93(10):768-83. doi: 10.1007/s00392-004-0137-6. PMID: 15492892. Helfant, R. H., et al. “Coronary Heart Disease. Differential Hemodynamic, Metabolic, and Electrocardiographic Effects in Subjects with and without Angina Pectoris during Atrial Pacing.” Circulation, vol. 42, no. 4, 1 Oct. 1970, pp. 601–610, www.ncbi.nlm.nih.gov/pubmed/11993303., https://doi.org/10.1161/01.cir.42.4.601. Takase, B., Kurita, A., Noritake, M., Uehata, A., Maruyama, T., Nagayoshi, H., ... & Nakamura, H. (1992). Heart rate variability in patients with diabetes mellitus, ischemic heart disease, and congestive heart failure. Journal of electrocardiology, 25(2), 79-88. Sroka, K., Peimann, C. J., & Seevers, H. (1997). Heart rate variability in myocardial ischemia during daily life. Journal of electrocardiology, 30(1), 45-56. Scheuer, J., & Brachfeld, N. (1966). Coronary insufficiency: relations between hemodynamic, electrical, and biochemical parameters. Circulation Research, 18(2), 178-189. Schmid, P. G., Greif, B. J., Lund, D. D., & Roskoski Jr, R. O. B. E. R. T. (1978). Regional choline acetyltransferase activity in the guinea pig heart. Circulation Research, 42(5), 657-660. Katz, A. M. (1971). Effects of ischemia on the cardiac contractile proteins. Cardiology, 56(1-6), 276-283. Manunta, Paolo, et al. “Endogenous Ouabain in Cardiovascular Function and Disease.” Journal of Hypertension, vol. 27, no. 1, 1 Jan. 2009, pp. 9–18, journals.lww.com/jhypertension/Abstract/2009/01000/Endogenous_ouabain_in_cardiovascular_function_and.3.aspx, https://doi.org/10.1097/HJH.0b013e32831cf2c6. Doepp, Manfred. “May Strophanthin Be a Valuable Cardiac Drug ? .” American Journal of Medical and Clinical Research & Reviews, vol. 2, no. 9, 15 Sept. 2023, pp. 1–6, ajmcrr.com/index.php/pub/article/view/75/74, https://doi.org/10.58372/2835-6276.1069. Accessed 29 Mar. 2024. Thayer, J. F., Yamamoto, S. S., & Brosschot, J. F. (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International journal of cardiology, 141(2), 122-131. Recent breakthrough studies have shone a light on the intriguing link between our microbiome – the diverse community of microorganisms residing in our gut and mouth – and the secret to a longer, healthier life. Scientists have long suspected that our genes, environment, and internal factors like the microbiome play a role in determining how long we live, but the specifics remained elusive. Now, thanks to cutting-edge research, we're getting closer to unraveling the mysteries of longevity. In this groundbreaking exploration, scientists employed a sophisticated approach called Mendelian randomization (MR) to delve into the intricate relationships between the human microbiome and longevity. By analyzing genetic data from large cohorts, they uncovered some compelling associations that shed light on the microbial players in the quest for a longer life. The Gut Chronicles: Microbial Superstars and CulpritsThe gut microbiome, a bustling metropolis of bacteria, has been a focal point in the quest for longevity. The study identified certain gut microbes as potential champions in the battle against aging. Microbial heroes like Coriobacteriaceae, Oxalobacter, and the probiotic Lactobacillus amylovorus were found to be positively linked to increased odds of longevity. On the flip side, a few gut microbes emerged as potential antagonists, with names like Fusobacterium nucleatum, Coprococcus, Streptococcus, Lactobacillus, and Neisseria negatively associated with longevity. These microbial foes might have a role in determining how gracefully we age. Oral Health: More Than Just a Pretty SmileThe study didn't stop at the gut; it extended its gaze to the oral microbiome, a less-explored but equally important realm. The findings suggested a fascinating connection between the oral microbiome and longevity. Specific oral bacteria were identified as potential influencers in the longevity game. Interestingly, the research hinted at a lower gut microbial diversity among centenarians (diversity appears to lower with age), but no significant difference in their oral microbiota. This finding underscores the importance of tracking the movements of these beneficial microbes across different parts of the body for a longer and healthier life. Decoding the Genetic Blueprint for LongevityThe study leveraged Mendelian randomization to unravel the causality between the microbiome and longevity. This approach, using genetic variants as tools, allowed scientists to explore the potential causal links between specific microbial features and the length of our lives. The bidirectional analyses provided a wealth of information, not only pinpointing specific microbes associated with longevity but also revealing the microbial preferences of genetically longevous individuals. For instance, genetic predisposition to longevity correlated with a higher abundance of Prevotella and a lower abundance of Bacteroides, suggesting a potential link between dietary choices and a longer life. Microbes and Diseases: Unraveling the We The study didn't just stop at longevity; it ventured into the realm of diseases. Certain microbes associated with longevity were found to have correlations with specific diseases. For example, Coriobacteriaceae, linked to longevity, was significantly reduced in patients with heart failure, suggesting a potential protective role against cardiovascular diseases. This "microbiota—disease—longevity" axis provides a nuanced understanding of how our microbial companions might influence not only our lifespan but also our susceptibility to various health conditions. What's Next in the Quest for a Longer LifeWhile the study opens exciting new avenues, there are some limitations to consider. The identified causalities didn't all reach statistical significance due to the vast number of microbial features tested. However, the robustness of the findings was supported by the replication of several identified causal links in independent datasets. Moving forward, researchers aim to collect more comprehensive individual-level data, including microbiome profiles, genetics, socio-economic factors, behaviors, and environmental influences. This holistic approach will help tease apart the individual contributions of these factors to longevity. In conclusion, this pioneering study, using Mendelian randomization, has provided us with a roadmap to explore the intricate connections between our microbiome and the quest for a longer, healthier life. As we unlock the secrets hidden in our genes and microbes, we inch closer to personalized approaches for healthy aging and interventions that could extend our time on this planet. referencesLiu, Xiaomin, et al. “Mendelian Randomization Analyses Reveal Causal Relationships between the Human Microbiome and Longevity.” Scientific Reports, vol. 13, no. 1, 29 Mar. 2023, p. 5127, www.nature.com/articles/s41598-023-31115-8, https://doi.org/10.1038/s41598-023-31115-8.
Bupropion, originally named Amfebutamone, sold under the brand name Wellbutrin, is a medication commonly prescribed for the treatment of major depressive disorder (MDD), as is often used off-label for attention deficit hyperactivity disorder (ADHD), anxiety, obesity, and bipolar disorder. While it has demonstrated efficacy in addressing certain mental health issues, it is essential to examine the potential harms associated with its use, particularly considering its modulation of neurotransmitters like norepinephrine (NE) and dopamine. This article aims to shed light on the risks of Wellbutrin use, with a focus on its implications for pregnant or lactating women. Additionally, we'll explore the idea that the indications for Wellbutrin may stem from underlying nutrition and lifestyle factors rather than a deficiency of the medication. prescription trendsAs of 2021, Bupropion, maintained its position as the 18th most prescribed drug in the United States. With an estimated 29,099,445 prescriptions filled, it remains a widely utilized medication in the realm of psychiatric pharmaceuticals. This notable figure underscores the prevalence of its use in addressing various conditions, including depression and smoking cessation. The estimated number of patients in the United States receiving Bupropion in 2021 reached 6,412,363. This statistic reflects the significant impact and reach of Bupropion across diverse patient populations. Its popularity could be attributed to its purported effectiveness in managing depressive disorders, ADHD, anxiety, and aiding individuals in smoking cessation efforts. descriptionWellbutrin (bupropion hydrochloride), unlike any other antidepressant on the market, is chemically characterized as a monocyclic aminoketone, is chemically unrelated to tricyclic, tetracyclic, selective serotonin re‑uptake inhibitor, or other known antidepressant agents. Its structure closely resembles that of diethylpropion; it is related to phenylethylamines. It is designated as (±)-1-(3-chlorophenyl)-2-[(1,1-dimethylethyl)amino]-propanone hydrochloride. Bupropion hydrochloride powder is white, crystalline, and highly soluble in water. It has a bitter taste and produces the sensation of local anesthesia on the oral mucosa. There is documented interindividual variability - everyone responds differently. Neurotransmitter Modulation: The Double-Edged SwordWellbutrin functions by influencing the levels of neurotransmitters in the brain, primarily inhibiting the breakdown of norepinephrine and dopamine. While this mechanism contributes to its antidepressant effects, it also raises concerns about potential side effects and risks. Altering neurotransmitter levels can lead to a range of adverse effects, with the most common including:
Here are some of the possible harms and common side effects associated with Wellbutrin:
While the approach of inhibiting the reuptake of dopamine and norepinephrine aims to enhance the availability of these neurotransmitters in the brain, an imbalance can lead to adverse effects. Excessive levels or prolonged elevated concentrations of these neurotransmitters may contribute to overstimulation and disrupt normal neural signaling. Norepinephrine is a neurotransmitter involved in the body's "fight or flight" response, influencing heart rate and blood pressure. Medications that impact norepinephrine reuptake can lead to cardiovascular side effects, including increased heart rate and elevated blood pressure. Individuals with pre-existing cardiovascular conditions may be at a higher risk for complications. Altered levels of dopamine and norepinephrine can influence mood and behavior. In some cases, inhibiting reuptake may contribute to psychiatric symptoms such as anxiety, restlessness, or irritability. Balancing the desired therapeutic effects with potential adverse psychological consequences is a delicate consideration. Abruptly discontinuing medications that inhibit dopamine and norepinephrine reuptake can lead to withdrawal symptoms. These symptoms may include mood swings, fatigue, and cognitive disturbances. Additionally, some individuals may develop a dependence on these medications, requiring careful management to taper off gradually. Dopamine and norepinephrine play roles in regulating sleep and appetite. Disrupting these neurotransmitters can lead to sleep disturbances, insomnia, or changes in appetite. Monitoring and addressing these side effects are crucial for maintaining overall well-being during the course of treatment. The response to medications that inhibit dopamine and norepinephrine reuptake can vary widely among individuals. Factors such as genetic predispositions, existing medical conditions, and concurrent medications may influence how the body reacts to these interventions. Individualized treatment plans and close monitoring are essential components of responsible prescribing. Impact on Pregnant or Lactating WomenPregnancy and lactation introduce unique considerations when it comes to medication use. The use of Wellbutrin (bupropion) or any other medication during pregnancy requires careful consideration of potential risks and benefits. While studies on the safety of Wellbutrin during pregnancy are inconclusive, there is evidence suggesting a potential association with adverse outcomes, including preterm birth and low birth weight. Additionally, Wellbutrin and its metabolites are present in human breast milk excretions, raising concerns about its impact on nursing infants including alterations in neurotransmitters. Expectant or breastfeeding mothers should engage in thorough discussions with their healthcare providers to weigh the potential benefits against the risks and explore alternative treatment options. Here are some considerations regarding the potential harms of taking Wellbutrin during pregnancy:
Wellbutrin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It's essential for individuals taking Wellbutrin and considering pregnancy, or those who become pregnant while on the medication, to discuss their situation with healthcare professionals. Abruptly stopping antidepressant medication can lead to a recurrence of depressive symptoms, which may have its own set of risks during pregnancy. Healthcare providers will carefully weigh the potential risks and benefits based on the individual's mental health needs. In some cases, healthcare professionals may recommend continuing the medication, adjusting the dosage, or exploring alternative treatments. Pregnant individuals should inform their healthcare providers about their medication use and work collaboratively to make informed decisions that prioritize both maternal mental health and the well-being of the developing fetus. Inactive IngredientsWellbutrin is supplied for oral administration as 75‑mg (yellow‑gold) and 100‑mg (red) film‑coated tablets. Each tablet contains the labeled amount of bupropion hydrochloride and the inactive ingredients:
While the active ingredient in Wellbutrin plays a significant role in its pharmacological effects, it's important not to overlook the inactive ingredients in the formulation. While they make up a smaller portion of the overall product, their cumulative impact should not be underestimated, especially considering the frequent administration of the medication. Though often considered inert, inactive ingredients can still interact with the body in various ways, potentially influencing drug absorption, metabolism, and overall therapeutic efficacy. Moreover, individual sensitivities or allergic reactions to certain inactive ingredients can occur, further emphasizing the importance of evaluating the entire list of components. Even seemingly minor alterations in inactive ingredients can have profound implications, particularly when considering long-term usage. Over time, repeated exposure to these substances may contribute to cumulative effects or unexpected reactions. Therefore, comprehensive scrutiny of all ingredients, active and inactive alike, is essential for a thorough assessment of the safety profile of Wellbutrin and other pharmaceutical products. Impact of Food coloringThe vibrant hues that adorn our favorite processed foods often come from artificial food colorings, but behind the visual appeal lies a potential risk, particularly for pregnant or lactating women. Artificial food color usually contains petroleum and is manufactured in a chemical process that includes formaldehyde, aniline, hydroxides, and sulfuric acids. Most impurities in the food color are in the form of salts or acids. Sometimes lead, arsenic, and mercury may be present as impurities. The U.S. FDA is yet to study the effects of synthetic dyes on behavior in children. To date, there are numerous scientific articles highlighting the relationship of consumption of food colorings to the following health conditions:
Underlying these conditions are the documented mechanics of action that cause physiologic imbalance, which include:
Research has raised concerns about the consumption of food colorings and their potential adverse effects, including implications for conditions like ADHD, autism, and gastrointestinal dysfunction. There is a substantial amount of data exploring the connection between artificial food colorings and ADHD. Researchers suggest that certain artificial colorings may exacerbate hyperactivity and inattention in children with ADHD. Pregnant women, mindful of their diet's impact on fetal development, may choose to limit the intake of foods containing these colorings. The relationship between food colorings and autism spectrum disorders (and the underlying inflammation in the brain) is a topic of ongoing investigation. Meta-analysis have documented a link, emphasizing the need for caution, especially during pregnancy and lactation. As the developing brain is susceptible to external influences, limiting exposure to artificial additives becomes a consideration for expectant and nursing mothers. Artificial food colorings have been associated with gastrointestinal disturbances, ranging from discomfort to more severe issues. Pregnant women, already navigating changes in digestion due to hormonal shifts, may choose to minimize exposure to food colorings to promote digestive well-being during this crucial period. Potential Harms for Pregnant and Lactating Women:
For pregnant or lactating women concerned about the potential harms of food colorings, opting for a diet rich in whole, unprocessed foods becomes a valuable strategy. Choosing fruits, vegetables, and other naturally colorful sources can provide vibrant flavors without the need for artificial additives. While the connection between food colorings and adverse health outcomes is an area of ongoing research, pregnant and lactating women may choose to err on the side of caution. Prioritizing a diet that minimizes artificial additives and focuses on nutrient-dense, whole foods is a proactive step toward supporting both maternal and fetal health. Always consult with healthcare professionals for personalized guidance based on individual circumstances and health considerations. Impact of PEGPolyethylene glycol (PEG) is considered "generally recognized as safe" (GRAS) when used in specific contexts, but some individuals may experience adverse effects. The concerns that arise with the safety of consuming PEG are multifold, including:
Depending on manufacturing processes, PEGs may be contaminated with measurable amounts of ethylene oxide and 1,4-dioxane. The International Agency for Research on Cancer classifies ethylene oxide as a known human carcinogen and 1,4-dioxane as a possible human carcinogen. Ethylene oxide can also harm the nervous system and the California Environmental Protection Agency has classified it as a developmental toxicant based on evidence that it may interfere with human development. 1,4-dioxane is also persistent. In other words, it doesn’t easily degrade and can remain in the environment long after it is rinsed down the shower drain. 1,4-dioxane can be removed from cosmetics during the manufacturing process by vacuum stripping, but there is no easy way for consumers to know whether products containing PEGs have undergone this process.In a study of personal care products marketed as “natural” or “organic” (uncertified), U.S. researchers found 1,4-dioxane as a contaminant in 46 of 100 products analyzed. While carcinogenic contaminants are the primary concern, PEG compounds themselves show some evidence of genotoxicity and if used on broken skin can cause irritation and systemic toxicity. PEG itself is classified as expected to be toxic or harmful as mentioned on the Environment Canada Domestic Substance List. The industry panel that reviews the safety of cosmetics ingredients concluded that some PEG compounds are not safe for use on damaged skin (although the assessment generally approved of the use of these chemicals in cosmetics). Also, PEG functions as a “penetration enhancer,” increasing the permeability of the skin to allow greater absorption of the ingredients — including harmful ingredients. Researchers have observed that a large percentage of parents, caregivers, and practitioners described an explosion of neurological side effects seemingly correlated to polyethylene glycol administration. Those side effects include:
Commonly found in various products, such as medications, laxatives, and skincare items, PEG may lead to the following potential harms:
People with pre-existing health conditions, especially those affecting the kidneys, should inform their healthcare providers before using PEG-containing products. As with any substance, individual responses to PEG can vary, and people experiencing adverse effects should seek medical attention promptly. It's important to weigh the risks and benefits of using PEG-based products based on individual health conditions and circumstances. Impact of TalcTalc is a mineral commonly used in various products such as talcum powder, cosmetics, and personal care items. While talc is considered GRAS for external use, there have been concerns and controversies regarding potential health risks associated with its use, primarily when used in certain ways or in specific product formulations. Here are some of the concerns related to talc:
Individuals concerned about the potential risks of talc-containing products should consider alternatives. As with any substance, moderation and careful use are advisable, and individuals experiencing adverse effects should seek medical advice. Impact of titanium dioxideTitanium dioxide is a widely used pigment and additive in various products, including cosmetics, sunscreens, paints, and food items. While it is generally recognized as safe when used in approved applications, there are concerns about potential health risks associated with certain forms and uses of titanium dioxide. Here are some considerations:
It's important to note that regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA), have assessed the safety of titanium dioxide in approved uses. The permissible limits and specifications vary depending on the application. Consumers concerned about titanium dioxide can choose products with alternatives or consult with healthcare professionals. As research continues, regulatory agencies may update guidelines to ensure the safe use of titanium dioxide in various products. Root Causes vs. MedicationIt's crucial to recognize that the conditions for which Wellbutrin is prescribed—ADHD, anxiety, obesity, and bipolar disorder—may not be caused by a deficiency of Wellbutrin itself. Rather, these conditions are complex and multifaceted, often influenced by underlying nutrition and lifestyle factors. Addressing these root causes is fundamental to comprehensive and sustainable mental health care. Nutrition and Lifestyle Factors: Unveiling the Roots of DepressionDepression, a complex and pervasive mental health condition, often finds its roots in a myriad of factors, extending beyond the realm of neurochemistry to include nutrition and lifestyle elements. Understanding and addressing these underlying factors can play a pivotal role in comprehensive depression management. Here's a closer look at the nutrition and lifestyle aspects that contribute to this intricate mental health landscape: 1. Dietary Choices:
Nutrition and Lifestyle Factors: Off-Label Uses
DeprescriptionAs with many psychotropic medications, Wellbutrin (bupropion) requires careful consideration when discontinuing to minimize the risk of withdrawal symptoms. Abruptly stopping Wellbutrin, also known as going "cold turkey," can lead to uncomfortable and potentially distressing withdrawal symptoms. It is crucial to approach the discontinuation of Wellbutrin with a gradual tapering process, personalized to individual needs, to ensure a smoother transition. Withdrawal symptoms, often associated with sudden cessation of psychotropic drugs, can manifest as a range of physical and psychological discomforts. These may include dizziness, headaches, irritability, mood swings, insomnia, and flu-like symptoms. The severity and duration of withdrawal symptoms can vary from person to person. To mitigate the risk of withdrawal symptoms, the American Psychiatric Association recommends a tapering approach for all antidepressants, including Wellbutrin. Tapering involves gradually reducing the dosage over a specified period, allowing the body to adjust to the decreasing levels of the medication. It is paramount not to discontinue Wellbutrin without consulting your healthcare provider. Your doctor can create a personalized taper schedule based on factors such as the duration of your medication use, your current dosage, and any specific symptoms you may be experiencing. Tapering is typically done over 6 to 8 weeks to provide a gradual adjustment. Every individual responds differently to medication changes. Your doctor can tailor the taper schedule to your unique needs, ensuring a careful balance between minimizing withdrawal symptoms and maintaining mental health stability. If you begin to experience withdrawal symptoms during the tapering process, it is crucial to communicate promptly with your healthcare provider. They may need to reassess your taper schedule or make adjustments based on your symptoms. Restarting Wellbutrin can often alleviate withdrawal symptoms within a few days. The journey of tapering off Wellbutrin is a collaborative effort between you and your healthcare provider. Open communication about your experiences and any emerging symptoms allows for adjustments that prioritize your well-being throughout the process. In the realm of psychotropic medications, a thoughtful and gradual approach to discontinuation is key. Tapering off Wellbutrin under the guidance of your healthcare provider not only minimizes the risk of withdrawal symptoms but also ensures a smoother transition, prioritizing your mental health. Remember, your doctor is your ally in this process, and together, you can navigate the complexities of tapering to promote your overall well-being. ConclusionWhile Wellbutrin has proven efficacy in certain contexts, it's imperative to approach its use with caution, especially considering its potential impacts on neurotransmitter modulation. Pregnant or lactating women should consult their healthcare providers to make informed decisions based on their unique circumstances. Moreover, recognizing that the indications for Wellbutrin may be rooted in broader lifestyle and nutritional factors encourages a more comprehensive approach to mental health care. Collaborative discussions between patients and healthcare professionals can pave the way for personalized and holistic treatment plans that address the underlying causes of mental health conditions. references“Bupropion - Drug Usage Statistics, ClinCalc DrugStats Database.” Clincalc.com, 2021, clincalc.com/DrugStats/Drugs/Bupropion. Medical Expenditure Panel Survey (MEPS) 2013-2021. Agency for Healthcare Research and Quality (AHRQ), Rockville, MD. ClinCalc DrugStats Database version 2024.01.
“Wellbutrin: Package Insert / Prescribing Information.” Drugs.com, www.drugs.com/pro/wellbutrin.html. Starr P, Klein-Schwartz W, Spiller H, Kern P, Ekleberry SE, Kunkel S. Incidence and onset of delayed seizures after overdoses of extended-release bupropion. Am J Emerg Med. 2009 Oct;27(8):911-5. doi: 10.1016/j.ajem.2008.07.004. PMID: 19857406. Spiller, Henry A., et al. “Bupropion Overdose: A 3-Year Multi-Center Retrospective Analysis.” The American Journal of Emergency Medicine, vol. 12, no. 1, Jan. 1994, pp. 43–45, https://doi.org/10.1016/0735-6757(94)90195-3. Accessed 28 Nov. 2021. Bakthavachalu, Prabasheela, et al. “Food Color and Autism: A Meta-Analysis.” Advances in Neurobiology, vol. 24, 2020, pp. 481–504, pubmed.ncbi.nlm.nih.gov/32006369/#:~:text=Many%20families%20with%20autistic%20children, https://doi.org/10.1007/978-3-030-30402-7_15. Hussain, Sunny Z., et al. “Probable Neuropsychiatric Toxicity of Polyethylene Glycol: Roles of Media, Internet and the Caregivers.” GastroHep, vol. 1, no. 3, May 2019, pp. 118–123, https://doi.org/10.1002/ygh2.336. Sellaturay, Priya, et al. “Polyethylene Glycol–Induced Systemic Allergic Reactions (Anaphylaxis).” The Journal of Allergy and Clinical Immunology: In Practice, Oct. 2020, https://doi.org/10.1016/j.jaip.2020.09.029. “Drugs & Medications.” Webmd.com, 2019, www.webmd.com/drugs/2/drug-17118/polyethylene-glycol-3350-oral/details. Black RE, Hurley FJ, and Havery DC. “Occurrence of 1,4-dioxane in cosmetic raw materials and finished cosmetic products.” Int J PharJ AOAC Int. 84, 3 (May-Jun 2001):666-70. Brashear, A. et al. “Ethylene oxide neurotoxicity: a cluster of 12 nurses with peripheral and central nervous system toxicity.” Neurology 46, 4 (Apr 1996):992-8. California. EPA. Office of Environmental Health Hazard Assessment. Chemicals Known to the State to Cause Cancer or Reproductive Toxicity. February 5, 2010.https://www.oehha.org/prop65/prop65_list/files/P65single020510.pdf Environmental Health Association of Nova Scotia. Guide to Less Toxic Products.Halifax: EHANS, 2004. https://www.lesstoxicguide.ca/index.asp?fetch=personal#commo. OCA (Organic Consumer Association). 2008. Consumer alert. Cancer-causing 1,4-dioxane found in personal care products misleadingly branded as natural and organic. Available: https://www.organicconsumers.org/bodycare/DioxaneRelease08.cfm Wangenheim J and Bolcsfoldi G. “Mouse lymphoma L5178Y thymidine kinase locus assay of 50 compounds.” Mutagenesis 3, 3 (May 1988):193-205. Biondi O, Motta S, and Mosesso P. “Low molecular weight polyethylene glycol induces chromosome aberrations in Chinese hamster cells cultured in vitro.” _Mutagenesis_17, 3 (May 2002):261-4. Lanigan, RS (CIR Expert Panel). “Final report on the safety assessment of PPG-11 and PPG-15 stearyl ethers.” Int J Toxicol.20 Suppl 4 (2001):13-26 Cosmetic Ingredient Review. Ingredient Reports — Quick Reference Table (summarizing publications through Dec 2009). https://www.cir-safety.org/staff_files/PublicationsListDec2009.pdf Epstein, S with Fitzgerald, R. Toxic Beauty. Dallas: BenBella Books, 2009: 158-9. Chen, Tao , et al. “Genotoxicity of Titanium Dioxide Nanoparticles.” Journal of Food and Drug Analysis, vol. 22, no. 1, 1 Mar. 2014, pp. 95–104, https://www.sciencedirect.com/science/article/pii/S102194981400009X, https://doi.org/10.1016/j.jfda.2014.01.008. In an update to its 2007 scientific statement, the American Heart Association (AHA) emphasizes the significant and multifaceted benefits of resistance training (RT) on cardiovascular health. Contrary to the misconception that RT solely enhances muscle mass and strength, the statement highlights the favorable physiological and clinical effects of this form of exercise on cardiovascular disease (CVD) and associated risk factors. The scientific statement aims to provide comprehensive insights into the impact of RT, either alone or in combination with aerobic training, on traditional and nontraditional CVD risk factors. More is not always betterEpidemiological evidence suggests that RT is associated with a lower risk of all-cause mortality and CVD morbidity and mortality. Adults who participate in RT have ≈15% lower risk of all-cause mortality and 17% lower risk of CVD, compared with adults who report no RT. Approximately 30 to 60 minutes per week of RT is associated with the maximum risk reduction for all-cause mortality and incident CVD. Notice this "U" shape in the curve when examining the relationship between RT and morbidity and mortality. This curve suggests that some RT is clearly beneficial, but has the volume of RT increases past a certain point the benefits drop and it becomes harmful. The concept of a "biphasic response" is fundamental to understanding hormesis. It describes the characteristic dose-response relationship observed in hormetic processes, where a substance or stressor elicits opposite effects at low and high doses. The response can be visualized as a U-shaped or J-shaped curve, illustrating the beneficial effects at low doses and potential harm at higher doses. Benefits of RT on Traditional CVD Risk FactorsThe AHA's scientific statement underscores the positive influence of RT on traditional CVD risk factors, including blood pressure (BP), glycemia, lipid profiles, and body composition. Numerous studies indicate that engaging in RT is associated with reduced resting BP, improved glycemic control, and favorable alterations in lipid profiles, contributing to a lower risk of all-cause mortality and CVD morbidity. Despite recommendations suggesting 2 days per week of RT, only 28% of U.S. adults adhere to this guideline, highlighting the need for increased awareness and promotion. RT and resting blood pressureRT has demonstrated the ability to reduce resting BP across diverse populations, with notable benefits observed in individuals with prehypertension and hypertension. The mechanisms behind these benefits include enhancements in endothelial function, vasodilatory capacity, and vascular conductance. The reductions in BP achieved through RT are comparable to those achieved with antihypertensive medications. RT and GlycemiaRT shows promise in improving glycemia and insulin resistance, leading to a lower incidence of diabetes. The evidence suggests a nonlinear dose-response association, with up to 60 minutes per week of RT associated with the maximum risk reduction for diabetes. RT and Lipid ProfilesWhile the effect on lipid profiles is modest, RT results in favorable changes in high-density lipoprotein cholesterol, total cholesterol, and triglycerides. These improvements are more pronounced in older adults and those with elevated cardiometabolic risk. Rt, Body composition, and weightRT positively influences body composition by increasing lean body mass and reducing body fat percentage. It is particularly effective in overweight or obese individuals, contributing to increased metabolic rate and mitigating weight gain over time. Benefits of RT on Nontraditional CVD Risk FactorsIn addition to traditional risk factors, the scientific statement highlights the potential mechanisms by which RT positively affects nontraditional CVD risk factors. These include increased cardiorespiratory fitness, improved endothelial function, and potential benefits for sleep quality, psychological health, and well-being. The AHA's updated scientific statement reinforces the pivotal role of resistance training in cardiovascular health, providing a comprehensive overview of its impact on both traditional and nontraditional risk factors. As the evidence supporting RT's benefits continues to grow, the statement serves as a valuable resource for clinicians and public health professionals, offering practical strategies for promoting and prescribing resistance training to enhance cardiovascular health in diverse populations. ReferencesPaluch, Amanda E, et al. “Resistance Exercise Training in Individuals with and without Cardiovascular Disease: 2023 Update: A Scientific Statement from the American Heart Association.” Circulation, 7 Dec. 2023, https://doi.org/10.1161/cir.0000000000001189. Accessed 11 Dec. 2023.
Momma H, Kawakami R, Honda T, Sawada SS. Muscle-strengthening activities are associated with lower risk and mortality in major non-communicable diseases: a systematic review and meta-analysis of cohort studies. Br J Sports Med. 2022 Jul;56(13):755-763. doi: 10.1136/bjsports-2021-105061. Epub 2022 Feb 28. PMID: 35228201; PMCID: PMC9209691. Nasal Breathing: A Breath of Fresh Air for Cardiovascular Wellness – Insights from New Research2/4/2024 The leading cause of death in the United States is cardiovascular disease, and the risk of cardiovascular issues can be predicted by factors such as blood pressure, heart rate variability, blood pressure variability, and cardiac vagal baroreflex sensitivity. The interplay between the cardiovascular and respiratory systems is highlighted, with a particular emphasis on how respiration affects key prognostic cardiovascular variables. This study explores the impact of nasal breathing compared to oral breathing on cardiovascular health in young adults. Nasal breathing is associated with humidification, warming, and filtration of inhaled air, potentially leading to bronchodilation and improved breathing efficiency. While past research has shown nasal breathing to have positive effects on resting metabolic demands, its influence on cardiovascular markers is not well-understood. The primary hypothesis is that nasal breathing, as opposed to oral breathing, will result in decreased blood pressure, improved heart rate variability, reduced blood pressure variability, and increased cardiac vagal baroreflex sensitivity at rest. The study aims to contribute to the understanding of how breathing patterns influence prognostic cardiovascular variables, aligning with the broader interest in the impact of breathing pace and training on cardiovascular health. The secondary hypothesis focuses on the effects of nasal breathing during submaximal exercise. The expectation is that nasal breathing, by attenuating the ventilatory response and metabolic demands, will lead to reduced blood pressure responses, improved heart rate variability, and decreased blood pressure variability during exercise. This aspect is particularly relevant due to the association between elevated exercise blood pressure and the risk of developing hypertension and cardiovascular disease. FindingsThe study findings are summarized, focusing on the impact of nasal vs. oral breathing on physiological and subjective variables at rest and during exercise. At rest, nasal breathing is associated with lower mean and diastolic blood pressure, improved heart rate variability metrics, reduced LF/HF ratio, and lower ratings of perceived exertion (RPE) and breathlessness (RPB). However, it increased systolic blood pressure average real variability. During submaximal exercise, differences between nasal and oral breathing were observed for RPB, suggesting a modest effect on reducing breathlessness during acute exercise. The discussion delves into the potential clinical significance of these findings, particularly the reduction in diastolic blood pressure during nasal breathing at rest. The study suggests a greater parasympathetic to sympathetic dominance during nasal breathing, indicated by changes in frequency-domain metrics of heart rate variability. Although nasal breathing did not significantly affect beat-to-beat blood pressure variability, there is speculation about potential connections between respiratory variables and blood pressure changes, emphasizing the need for further investigation. The study notes that the impact of nasal breathing on cardiovascular variables may have implications for various populations and suggests avenues for future research, including examining nasal breathing's effects on blood pressure over longer durations, both at rest and during activities like exercise. The discussion also touches on the potential benefits of interventions like mouth-taping overnight, emphasizing the importance of considering nasal breathing in the context of broader health outcomes. In summary, the study highlights the potential benefits of nasal breathing, with improvements in various cardiovascular and subjective measures at rest. While the effects during exercise are more modest, the findings contribute to understanding the nuanced relationship between respiratory patterns and cardiovascular health. referencesWatso, Joseph C., et al. “Acute Nasal Breathing Lowers Diastolic Blood Pressure and Increases Parasympathetic Contributions to Heart Rate Variability in Young Adults.” American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, vol. 325, no. 6, 1 Dec. 2023, pp. R797–R808, pubmed.ncbi.nlm.nih.gov/37867476/, https://doi.org/10.1152/ajpregu.00148.2023.
Unveiling the Interplay Between Air Quality and Cardiometabolic Health: A Surprising Connection1/29/2024 In a groundbreaking study, researchers have delved into the intricate relationship between air quality and cardiometabolic health, revealing startling findings that challenge conventional wisdom. Published in Environmental Research, this research sheds light on the impact of air pollutants, even at concentrations below the World Health Organization's (WHO) 2021 guidelines, on various aspects of cardiovascular and metabolic well-being. Key FindingsThe study, conducted over a period of 33 weeks with 82 participants grappling with obesity, examined the associations between air pollutants and cardiometabolic outcomes. Particulate matter emerged as a significant player, demonstrating a strong connection with blood lipids, hormones, and glucose regulation – key markers of cardiometabolic health. Surprisingly, the research also uncovered a potential mitigating factor: diet. The participants' adherence to a Healthy Nordic diet, as measured by the Baltic Sea Diet score, showcased a remarkable ability to modify the impact of air pollution on certain cardiometabolic parameters. Details of the StudyThe study utilized linear mixed-effects models to analyze data gathered during a weight loss and weight loss maintenance intervention. The results revealed 17 significant associations between various air pollutants and 10 distinct cardiometabolic outcomes. The focus was primarily on blood lipids, hormones, and glucose regulation, providing a comprehensive understanding of the multifaceted effects of air pollution. Interestingly, the Baltic Sea Diet score did not appear to mediate the association between air pollution and cardiometabolic outcomes. However, the diet quality factor emerged as a key player in modifying the impact of particulate matter (PM2.5) on total cholesterol. Furthermore, it influenced the associations of nitrogen dioxide (NO) and ozone (O3) with ghrelin, a hormone associated with appetite regulation. ReferencesHealy, Darren R., et al. “Associations of Low Levels of Air Pollution with Cardiometabolic Outcomes and the Role of Diet Quality in Individuals with Obesity.” Environmental Research, vol. 242, 1 Feb. 2024, p. 117637, www.sciencedirect.com/science/article/pii/S0013935123024416, https://doi.org/10.1016/j.envres.2023.117637.
Navigating Hormesis: Embracing the Balance Between Stress and Strength for Optimal Health12/21/2023 In the intricate dance of biological responses to stress, hormesis emerges as a captivating phenomenon, challenging conventional notions of dose-response relationships. This blog post delves into the fascinating world of hormesis, where the subtle interplay between stressors and adaptive responses shapes our understanding of health and resilience. The Hormetic Curve: Unveiling the U-Shaped StoryExplore the dynamics of hormesis through the lens of a U-shaped or J-shaped curve, where low doses of stressors trigger beneficial responses while high doses lead to toxicity. Understand how this nonlinear relationship challenges traditional toxicological paradigms. Small Doses, Big Impact: Hormetic Responses UnveiledDelve into real-world examples of hormetic responses, from the beneficial effects of low-dose radiation to the adaptive mechanisms activated by moderate exercise. Learn how these responses stimulate resilience, adaptation, and overall well-being. Hormesis in Toxicology: Redefining Risk AssessmentUnravel the complexities of hormesis in toxicology, where the concept challenges established risk assessment practices. Discover how hormesis introduces a nuanced understanding of dose-dependent effects, prompting a reevaluation of regulatory frameworks. Hormesis and Adaptive Learning: "What Doesn't Kill You Makes You Stronger"Connect the dots between hormesis and the age-old adage, "What doesn't kill you makes you stronger." Explore how the hormetic principle aligns with the idea that controlled exposure to stressors can lead to adaptive learning, fostering strength and resilience. Balancing Act: The Importance of ModerationEmphasize the crucial role of balance and moderation in hormesis. Uncover the delicate equilibrium required for stressors to act as catalysts for positive adaptation without tipping into harmful territory. From Sirtuins to Telomeres: Hormesis at the Cellular LevelJourney into the cellular realm and discover how hormesis influences sirtuins, telomeres, and other cellular processes. Explore the implications for cellular repair, mitochondrial function, and the potential impact on longevity. Practical Insights: Applying Hormesis in Everyday LifeGain practical insights into how hormesis can be applied in daily life. Explore lifestyle choices, dietary considerations, and stress management techniques that harness the power of hormetic responses for enhanced well-being. Embark on a journey of discovery as we unravel the layers of hormesis, revealing its impact on biology, health, and our quest for a balanced and resilient life. Embrace the science behind stress and strength, and learn how hormesis invites us to rethink our approach to well-being.
The documentary "War on Ivermectin" explores the contentious landscape surrounding the drug Ivermectin during the COVID-19 pandemic. It delves into the controversy surrounding the use of Ivermectin as a potential treatment for COVID-19 and the challenges it has faced from regulatory bodies and mainstream medical establishments.
The documentary presents perspectives from advocates of Ivermectin, who argue for its efficacy and safety in treating COVID-19. It may shed light on the resistance faced by proponents of Ivermectin, exploring factors such as media portrayal, regulatory decisions, and the broader implications for the treatment landscape. Additionally, the documentary might feature interviews with medical professionals, researchers, and individuals who have been affected by the debate over Ivermectin. It aims to provide a comprehensive overview of the complex and polarized discussions surrounding the drug in the context of the global response to the pandemic.
"The Business of Being Born" is a documentary that explores the maternity care system in the United States, shedding light on the medicalization of childbirth and advocating for a more holistic and woman-centered approach. Produced by Ricki Lake and Abby Epstein, the film challenges conventional hospital birthing practices and highlights the benefits of midwifery and home births.
The documentary features interviews with midwives, doctors, and mothers who share their experiences and perspectives on childbirth. It addresses concerns about the rising rates of cesarean sections, interventions, and the impact of hospital protocols on the birthing process. The film advocates for informed decision-making, empowering women to make choices that align with their preferences and needs during childbirth. Through personal stories and expert opinions, "The Business of Being Born" encourages viewers to rethink the way society approaches and views childbirth, urging a return to more personalized, woman-centered, and natural birthing experiences. The documentary contributes to the ongoing conversation about maternity care practices and promotes awareness of alternative options for expectant mothers. Women are not the only ones who experience hormonal changes in midlife. Men also undergo a transition similar to menopause called andropause, also known as late-onset hypogonadism (LOH), during which vitality hormones, particularly testosterone (T), but also human growth hormone (HGH), are produced in lower quantities. As men age, not only does the body start making less testosterone, but also the levels of another hormone called sex hormone binding globulin (SHBG), which pulls usable testosterone from the blood, begins to increase. Andropause is a natural phase of aging, but is accentuated by many factors including, but not limited to age-associated comorbid illnesses, medications, and malnutrition. The age at which symptoms of andropause may manifest can vary, but it typically occurs in middle-aged and older men, beginning in the late 40s to early 50s, with the most frequent age occurring between 51-60 years, with patients reporting symptoms such as impotence, weakness, and memory loss. Other age-related alterations due to andropause include body composition, mood, cognitive function, sleep, and erythropoiesis. The pharmaceutical industry has capitalized heavily on this 'change of life' phase, with Viagra, among other pharmaceuticals, but these pharmaceuticals have severe, if not sometimes deadly side effects. All the more reason why modifiable factors and natural alternatives are in great need today. While the process of andropause is considered inevitable, understanding the causes and adopting proactive nutrition and lifestyle strategies can prevent an early onset of this condition, and significantly alleviate symptoms thereby enhancing overall quality of life. normalization of Low TTestosterone is a crucial hormone for both men and women, contributing to muscle bulk, strength, fat-burning, and overall vitality. Adequate testosterone levels provide a chiseled look, high energy, and strength in men, and definition, muscle, and energy in women. However, declining testosterone levels can lead to increased fatigue, difficulty building muscle, and higher fat accumulation, posing a risk to overall health. Contrary to the common belief that decreasing testosterone is solely an aging-related issue, it has been observed that testosterone levels, even in young men, have been declining for decades. Factors contributing to this decline include the Standard American Diet, characterized by high sugar intake, imbalanced omega-6 to omega-3 ratios, processed foods lacking essential nutrients, and the attack on cholesterol. Additionally, environmental toxins play a role in lowering testosterone levels. Notably, the Standard American Diet, abundant in sugars, particularly processed sugars and carbohydrates, elevates cortisol levels, which inversely affects testosterone. The imbalance of omega-6 to omega-3 ratios, primarily due to the prevalence of corn and soy in processed foods, further contributes to cortisol elevation, adversely impacting testosterone levels. Processed foods with low nutritional value, combined with the demonization of cholesterol, essential for testosterone production, also play a role in this decline. Addressing these issues through dietary adjustments, such as choosing organic and grass-fed options, avoiding processed foods and sugars, ensuring adequate protein intake, and engaging in regular exercise, can positively impact testosterone levels and overall health. Understanding and addressing these lifestyle factors is essential for maintaining optimal hormonal balance and supporting longevity. PSYCHONEUROIMMUNOLOGY: |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Acid Blockers |
ADHD drugs |
Adjuvant |
Adderall |
Adrenaline |
Aluminum |
Antidepressants |
Antihypertensive drugs |
Antipsychotic drugs |
Antiretroviral drugs |
Atorvastatin |
Benzophenones |
Bile Acid Sequestrants (+ binding resins) |
Bisphenols (BPA, BPF, BPS) |
Cell Phone Exposure |
Cesium-137 |
Cholesterol Lowering Drugs |
Concerta |
Corticosteroid |
Dexamethasone |
Electronic Cigarettes |
Ethinyl Estradiol (plus Lynestrenol) |
Ethylene Glycol |
Fluoride |
Fructose |
Gluten (and Exorphins) |
Hexachlorocyclohexane |
Histamine Receptor Antagonists |
Ibuprofen |
Infant Formula |
Lead |
Levonorgestrel/ethinyl estradiol |
Lovastatin |
Mercury |
Monosodium Glutamate (MSG) |
Mycoestrogens |
Nanoparticles |
Nonylphenol [and Ethoxylate (NPE)] |
Oral Contraceptives |
Organochlorine Pesticides & Compounds |
Organophosphate Pesticides |
Persistent Organic Pollutants (POPs) |
Pesticides |
Phenothrin |
Polybrominated Diphenylethers (PBDEs) |
Polyoxyethylene Amine |
Prednisone |
Pravastatin |
Progestins |
Rosuvastatin |
Simvastatin |
Sodium Fluoride |
Soy |
Statin Drugs |
Sugar Sweetened Beverages |
Tamoxifen |
Thimerosal |
Thiazide Diuretics |
Tin |
Titanium Nanoparticles (including Dioxide) |
Tween 80 (Polysorbate 80) |
Vinclozolin |
Vitamin A Palmitate |
Zearalenone (ZEA) |
It's important to be aware of potential exposure to these substances and take steps to minimize risks. This includes choosing products that are labeled as BPA-free, using natural and organic personal care products, and being mindful of pesticide residues on food. Additionally, maintaining a healthy lifestyle, including a balanced diet and regular exercise, can help support overall endocrine health.
Bisphenol-A (BPA)
The mechanism by which BPA may lead to lower testosterone levels involves its ability to mimic or interfere with the action of hormones. BPA is known to have estrogenic properties, meaning it can bind to estrogen receptors in the body, thereby mimicking the effects of estrogen. This can disrupt the delicate hormonal balance, leading to alterations in the normal regulatory processes of the endocrine system.
Excessive estrogenic activity, whether from BPA or other sources, can negatively impact the production of testosterone. Estrogen and testosterone are usually balanced in the body, and disruptions in this balance can lead to a decrease in testosterone levels. BPA may interfere with the function of Leydig cells in the testes, which are responsible for producing testosterone. This interference can result in reduced testosterone synthesis.
BPA may interfere with the signaling pathways involved in hormone production and regulation. This disruption can lead to a cascade of effects, including reduced stimulation of testosterone production by luteinizing hormone (LH) from the pituitary gland.
BPA exposure has been associated with testicular abnormalities, including changes in testicular morphology and function. These changes can contribute to lower testosterone levels.
It's important to note that the impact of BPA on testosterone levels can be influenced by factors such as the duration and level of exposure, individual sensitivity, and overall health. Chronic exposure to BPA, particularly during critical developmental periods, may have more pronounced effects. Reducing exposure to BPA by using BPA-free products, choosing fresh foods over canned goods, and being mindful of plastic usage may help mitigate potential risks.
Smoking
Leydig cells in the testes are responsible for producing testosterone. Smoking exposes the body to various harmful chemicals, including those in cigarette smoke, and glycerin. These toxic substances can directly affect Leydig cells, leading to dysfunction and a decrease in testosterone production.
Smoking generates oxidative stress in the body due to the production of free radicals and reactive oxygen species. Oxidative stress has been linked to damage to testicular cells, including Leydig cells. This damage can interfere with the normal process of testosterone synthesis.
Smoking is known to constrict blood vessels and impair blood flow. This vasoconstriction can affect blood supply to the testes, compromising their function. Inadequate blood flow to the testes may contribute to decreased testosterone production.
Smoking can disrupt the delicate balance of hormones involved in reproductive health. For example, it may lead to an increase in cortisol, a stress hormone, which can negatively influence testosterone levels. Hormonal imbalances, particularly elevated stress hormones, can interfere with the normal regulatory mechanisms of testosterone production.
Smoking has been associated with increased aromatase activity. As mentioned, higher aromatase activity can lead to a greater conversion of testosterone to estrogen, resulting in lower testosterone levels.
Smoking has been linked to structural damage in the testes. This damage can impact the overall health of testicular tissue and contribute to lower testosterone levels.
Luteinizing hormone (LH) stimulates the production of testosterone by the Leydig cells. Smoking has been associated with decreased levels of LH. Reduced LH levels can result in diminished stimulation of testosterone production.
It's important to note that the impact of smoking on testosterone levels can vary among individuals, and factors such as the duration and intensity of smoking, overall health, and genetic predisposition may influence the extent of the effect. Quitting smoking is a crucial step in promoting overall health, including reproductive health, and may contribute to the restoration of normal testosterone levels over time.
Alcohol
Chronic alcohol consumption has been linked to testicular atrophy, which is a reduction in the size and function of the testes. Testicular atrophy may result in a decreased ability of Leydig cells (which produce and respond to testosterone) to function optimally, leading to lower testosterone levels.
Alcohol can disrupt the normal hormonal regulation of the endocrine system. Chronic alcohol use may alter the balance of hormones involved in reproductive health. Alcohol consumption has been associated with increased cortisol levels (a stress hormone), which can have inhibitory effects on testosterone production.
Alcohol can suppress the release of GnRH, a hormone that stimulates the production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary gland. Reduced GnRH levels may lead to lower LH levels, which, in turn, can diminish the stimulation of testosterone production by Leydig cells.
Chronic alcohol consumption has been associated with increased aromatase activity. Elevated aromatase activity can lead to a greater conversion of testosterone to estrogen, resulting in lower testosterone levels.
The liver is involved in the metabolism of hormones, including testosterone. Chronic alcohol use can lead to liver damage and impaired liver function. Liver dysfunction may impact the normal clearance and metabolism of hormones, potentially contributing to hormonal imbalances, including lower testosterone levels.
Alcohol interferes with the absorption and utilization of certain nutrients, including zinc. Zinc is an essential mineral for testosterone production. Nutrient deficiencies, particularly zinc deficiency, may contribute to decreased testosterone synthesis.
Excessive alcohol consumption can disrupt sleep patterns. Sleep is crucial for the natural production of testosterone during the night. Poor sleep quality or insufficient sleep may negatively impact testosterone levels.
It's important to note that the impact of alcohol on testosterone levels can vary among individuals, and factors such as the amount and duration of alcohol consumption, overall health, and genetic predisposition may influence the extent of the effect. Moderation in alcohol consumption and maintaining a healthy lifestyle are important considerations for overall well-being, including reproductive health.
Excess Environmental Heat (sauna)
- Improved Circulation and Blood Flow: Improved blood flow may enhance nutrient and oxygen delivery to tissues, including the testes, potentially supporting optimal Leydig cell function responsible for testosterone production.
- Heat Stress and Hormetic Response: Hormetic stressors, including heat stress from sauna use, may stimulate the release of certain hormones, potentially influencing the endocrine system, including testosterone regulation.
- Stress Reduction and Cortisol Modulation: Chronic stress and elevated cortisol levels have been linked to disruptions in testosterone balance. Sauna-induced relaxation may help modulate cortisol levels and support hormonal balance.
- Detoxification and Elimination of Toxins: Some environmental toxins may interfere with hormone balance, and the elimination of these toxins through the skin may indirectly support hormonal health, including testosterone levels.
- Enhanced Recovery and Exercise Benefits: Regular exercise is associated with improved testosterone levels. Sauna acts as a non-impact cardio sessions, and especially when performed post-exercise may enhance recovery, potentially supporting the overall positive effects of exercise on hormonal health.
- Improved Sleep Quality: Sauna use, particularly in the evening, has been reported to promote relaxation and improve sleep quality. Quality sleep is crucial for the natural regulation of hormones, including testosterone. Improved sleep may indirectly contribute to hormonal balance.
- Cardiovascular Health Benefits: Sauna use has been associated with cardiovascular benefits, including improved endothelial function and reduced blood pressure. Cardiovascular health is linked to overall well-being, and maintaining a healthy cardiovascular system may positively influence hormonal balance.
Given the notable benefits of sauna, it has been demonstrated that prolonged exposure to excessive heat, such as in hot environments or the use of hot baths and saunas, has been associated with potential impairment of testosterone levels. Several mechanisms may contribute to the negative impact of heat on testosterone.
The testes are located outside the body in the scrotum, a sac of skin. This positioning is crucial for maintaining a lower temperature than the core body temperature, which is necessary for optimal sperm and testosterone production. Prolonged exposure to heat, especially if the testes are subjected to elevated temperatures, can disrupt the normal temperature regulation and impair the function of Leydig cells, which produce testosterone.
Elevated testicular temperatures resulting from prolonged exposure to heat have been linked to a decrease in sperm production (spermatogenesis). The same conditions that inhibit spermatogenesis may also impact Leydig cell function, leading to a reduction in testosterone synthesis.
GnRH stimulates the release of luteinizing hormone LH from the pituitary gland. LH, in turn, stimulates the Leydig cells to produce testosterone. Prolonged heat exposure has been associated with a decrease in GnRH and LH levels, potentially leading to reduced stimulation of testosterone production.
The body perceives excessive heat as a stressor, leading to the activation of the stress response, including the release of cortisol. Elevated cortisol levels can negatively impact the balance of sex hormones, potentially suppressing testosterone synthesis. While sauna has been observed to lower cortisol, perception and experience are important to note. In other words, if an individual does not have much experience with deliberate heat exposure and decides to enter extreme heat for long durations, their body likely cannot compensate to the stressor. Gradual progressions in intensity and durations for sauna use are recommended.
High temperatures can affect the quality of sperm, leading to decreased motility and fertility. The relationship between impaired sperm quality and testosterone levels suggests that the detrimental effects of heat may extend to Leydig cell function and testosterone synthesis.
It's important to note that the impact of heat on testosterone levels can vary among individuals, and the body's ability to regulate temperature may differ. Additionally, the body has mechanisms to cope with short-term variations in temperature. However, chronic or extreme heat exposure may pose risks to reproductive health.
Non-native EMFs
A comprehensive review of studies from 2003 to 2020 highlighted several key findings:
- Sperm Quality: Both human and animal studies indicate that exposure to EMR from mobile phones leads to reduced sperm motility, structural anomalies, and increased oxidative stress due to overproduction of reactive oxygen species (ROS).
- Semen Analysis: Human semen samples exposed to mobile phone EMR showed significant reductions in motility and viability, and increased ROS levels. Laboratory-controlled exposure of semen samples to mobile phone radiation resulted in decreased sperm concentration and quality.
- Epidemiological Studies: Research involving large cohorts of men demonstrated a correlation between mobile phone usage and lower semen volume, sperm concentration, and total sperm count. Constant use of mobile internet services was particularly linked to poorer sperm quality.
- Survey Results: Surveys of men referred for semen analysis revealed that prolonged phone usage (over an hour per day) and usage while charging were associated with higher percentages of abnormal sperm concentrations.
- Experimental Findings: Studies on male rats exposed to mobile phone radiation showed slight decreases in serum testosterone levels and testicular weight. Other experiments demonstrated that EMR exposure led to genotoxic effects on spermatozoa and altered pituitary function, affecting the Leydig and Sertoli cells critical for male fertility.
- Laptop Exposure: Sperm samples exposed to Wi-Fi radiation from laptops for extended periods showed reduced motility and increased DNA fragmentation.
- Military Study: An increased rate of childlessness was observed among military men exposed to RF electromagnetic fields (EMF), further supporting the link between EMR exposure and male infertility.
Here are some proposed mechanisms through which exposure to non-native EMFs, such as Wi-Fi, Bluetooth, cellphones, and sources of "dirty electricity" including electric-generated heaters, negatively influence testosterone levels:
- Scrotal Hyperthermia and Oxidative Stress: These were identified as primary mechanisms through which EMR affects male fertility. Long-term and frequent use of mobile phones exacerbates these effects.
- Increased Oxidative Stress: Exposure to EMFs has been associated with increased oxidative stress in some studies. Oxidative stress refers to an imbalance between free radicals and antioxidants in the body. Elevated oxidative stress may have the potential to disrupt the endocrine system, including the regulation of testosterone.
- Disruption of Melatonin Production: EMF exposure, especially from devices used at night like cellphones, may interfere with melatonin production. Melatonin is a hormone that regulates sleep-wake cycles. Disruption of melatonin levels can impact the circadian rhythm and potentially influence testosterone production, as testosterone follows a circadian pattern with higher levels during sleep.
- Alteration of Calcium Ion Movement: Researchers have observed that non-native EMFs increase the movement of calcium ions in cells. Calcium ions play a role in various cellular processes, including hormone production. Disruption of calcium ion movement influences signaling pathways involved in testosterone synthesis, among other harmful effects.
- Impact on Leydig Cells: As mentioned, Leydig cells in the testes are responsible for producing testosterone. Researchers have observed exposure to EMFs affects Leydig cell function, thereby altering testosterone synthesis.
- Heat Generation: Certain devices emitting EMFs, especially those with high power, may generate heat. Prolonged exposure to localized heat, particularly in the groin area where the testes are located, could potentially impact sperm quality and testosterone production
Environmental Toxins
- Endocrine Disruption: Many environmental toxins are classified as endocrine-disrupting chemicals (EDCs). These substances can mimic or interfere with the actions of hormones, including testosterone. EDCs may bind to hormone receptors, blocking or activating them inappropriately. This interference can lead to imbalances in hormonal signaling, including the regulation of testosterone production.
- Aromatase Activity and Estrogen Dominance
- Disruption of Leydig Cell Function
- Inhibition of Gonadotropins: Some environmental toxins can interfere with the secretion of gonadotropins, such as LH and FSH, which regulate testosterone production. Inhibition of gonadotropin release may lead to diminished stimulation of Leydig cells and, consequently, lower testosterone levels.
- Testicular Toxicity: Certain environmental toxins may exhibit testicular toxicity, causing damage to the testes and impairing their function. Testicular damage can impact Leydig cell activity and overall testosterone synthesis.
- Oxidative Stress: Some environmental toxins can induce oxidative stress, resulting in an imbalance between free radicals and antioxidants in the body. Oxidative stress has been associated with testicular damage and impaired testosterone production.
- Impaired Sperm Quality: Environmental toxins may affect sperm quality, including motility and morphology. Sperm abnormalities can be indicative of disruptions in the testicular microenvironment, potentially influencing testosterone levels.
- Epigenetic Changes: Exposure to environmental toxins may lead to epigenetic changes, alterations in gene expression without changes to the underlying DNA sequence. Epigenetic modifications in genes related to testosterone synthesis and regulation can impact hormonal balance.
Below is a list of known compounds, chemicals, and environmental toxins that reduce testosterone levels, as supported by scientific literature:
Anti-Androgens |
Atorvastatin |
Bisphenol A |
Ethinyl Estradiol (plus Lynestrenol) |
Glyphosate (Roundup |
Ibuprofen |
Levonorgestrel / ethinyl estradiol |
Lovastatin |
Organophosphate pesticides |
Parabens |
Pesticides |
Phthalates |
Simvastatin |
Statin Drugs |
Sugar Sweetened Beverages |
Titanium Dioxide |
Titanium Nanoparticles |
Vitamin A Palmitate |
Solutions to andropause
It's important to note that individual responses to lifestyle interventions can vary, and results may take time.
Nutritional Strategies
Here is an evidence-based list of foods, compounds, and substances known to enhance testosterone levels:
Astragalus |
Astaxanthin |
Bitter Melon |
Biochanin A |
Caffeine |
Calcium* |
Coconut (+ Oil) |
Coleus Forskohlii |
Curcumin |
Daidzein |
Dogwood |
Fermented Foods and Beverages |
Formononetin |
Genistein |
|
Ginseng (Korean) |
Ginsenosides |
Ginkgo biloba |
Isoflavones |
Linoleic acid^ (Conjugated) |
Maca |
Molybdenum |
Mulberry |
||
Olive |
Onion |
Pantothenic Acid (Vitamin B-5) |
Phosphatidylserine |
Phytoestrogens (+/-) |
Raspberry |
Resveratrol |
Saffron |
Selenium |
|
Squalene |
Suma (Pfaffia Paniculata) |
Taro |
Tauroursodeoxycholic acid |
|
Vitamin E |
Zinc |
Before embarking on a supplement regimen, prioritize the quality of ingredients. Opt for reputable brands that use high-quality, pure ingredients. The effectiveness and safety of a supplement are inherently linked to the quality of the components it contains.
Understanding the appropriate dosage for each compound is paramount. Dosage recommendations can vary based on factors such as age, health status, and individual response. Always follow recommended dosages and, if uncertain, consult with a healthcare professional for personalized advice.
Enhancing testosterone is not just about isolated compounds; it's a holistic journey. Lifestyle factors, including nutrition, exercise, sleep, and stress management, play pivotal roles in hormonal balance. Consider adopting a well-rounded approach that encompasses these lifestyle elements.
Before introducing additional compounds, it's wise to address and minimize harmful stressors in your life. Chronic stress, inadequate sleep, and poor dietary choices can negatively impact hormone levels. Individuals may find more significant benefits by first focusing on stress reduction and overall well-being.
It's essential to recognize that responses to supplements can vary widely among individuals. What works for one person may not yield the same results for another. Pay attention to how your body responds, and be patient; changes may take time.
*^The hormonal system is complex and nuanced. Just because researchers have observed the calcium and linoleic acid (LA) can increase testosterone, more is not better. Excess LA (an essential fatty acid - the body cannot make it, and must consume it in the diet) is well established to disrupt metabolic function, which can thereby lead to lower testosterone. Innumerable amounts of food contain LA, therefore to call it "essential" can be deceiving. It is important to note that both calcium and LA should be consumed in whole food sources.
In conclusion, while compounds like zinc, magnesium, and various herbs have been associated with potential testosterone support, a thoughtful and informed approach is crucial. Prioritize high-quality ingredients, determine suitable dosages, and consider the broader lifestyle factors influencing hormonal health. Taking proactive steps to reduce harmful stressors can set a solid foundation for any testosterone-enhancing efforts. Before making significant changes to your supplement routine, it's advisable to consult with healthcare professionals for personalized guidance tailored to your unique needs. Remember, optimizing testosterone is a holistic endeavor that encompasses both supplementation and a balanced, healthy lifestyle.
Aromatase inhibitors
Aromatase inhibitors are often utilized in medical scenarios where reducing estrogen levels is crucial, such as in the treatment of hormone-sensitive breast cancer in postmenopausal women. In men undergoing TRT, aromatase inhibitors may be used to manage or prevent symptoms of estrogen dominance that can occur with exogenous testosterone administration. Aromatase inhibitors should be used judiciously, as completely suppressing estrogen levels in men can have adverse effects on bone health, libido, and overall well-being. Additionally, as paradoxical as it might sound, some aromatase inhibitors are actually estrogenic (e.g. anastrozole). The use of aromatase inhibitors should be tailored to individual needs, and regular monitoring of hormone levels is essential to ensure a balanced hormonal profile.
There are many natural aromatase inhibitors including progesterone, Maca, Grape seed extract, Nettle, Saw Palmetto, and more. The following foods contain compounds that have been shown to inhibit aromatase activity, thereby suppressing estrogen biosynthesis:
In summary, aromatization is a natural and complex process with essential physiological functions. However, imbalances in aromatase activity can have implications for hormonal health. Aromatase inhibitors, when used under medical supervision, can help manage hormonal imbalances and associated symptoms. It's crucial to approach hormone management with a comprehensive understanding of individual health needs and regular monitoring.
Artichokes |
Arugula |
Black Tea |
Blueberries |
Broccoli |
Brussel Sprouts |
Cabbage |
Cauliflower |
Celery |
Cherries |
Chives |
Cilantro |
Collard Greens |
Corn |
Cranberries |
Ligonberries |
Currants |
Bilberries |
Grapes |
Green Onions |
Green Tea |
Honey (Raw) |
Horseradish |
Peppers |
Lemons & Limes |
Mexican Oregano |
Mushrooms |
Mustard |
Mustard Greens |
Oats |
Oranges |
Parsley |
Passion Fruit |
Pomegranates |
Radishes |
Saffron |
Turnips |
Turnip Greens |
Walnuts |
Watercress |
Exercise
1. Resistance Training: Regular engagement in resistance or strength training exercises holds a pivotal role in sustaining hormonal equilibrium. Compound movements like squats, deadlifts, and weightlifting emerge as catalysts for increased testosterone production. By activating large muscle groups, such as incorporating lower-body exercises like squats and lunges alongside upper-body workouts, trigger a substantial hormonal response, elevating testosterone levels.
Heavy lifting, especially with full-body exercises like squats, deadlifts, and bench presses, is crucial for boosting testosterone. Use weights at 85-95% of your one-rep max (1RM) and aim for 2-3 full-body workouts per week. Beginners can start with weight machines before transitioning to free weights.
Longer rest periods (around 120 seconds) between sets are better for testosterone production. To make the most of your time, alternate between exercises that don't stress the same muscles. For example, pair bench presses with squats, taking shorter breaks between each.
Forced reps involve performing as many reps as possible, then having a partner assist with a few additional reps. This method has been shown to increase testosterone more effectively than solo reps. Incorporate forced reps into the last set of your exercises.
2. High-Intensity Interval Training (HIIT): HIIT workouts introduce brief, intense bursts of exercise followed by rest or lower-intensity periods. Integrating HIIT into your routine exhibits positive effects on testosterone levels. The dynamic nature of HIIT prompts the body's adaptive response, nurturing hormonal balance. Studies show that short, intense sprints can significantly boost testosterone levels. For optimal results, perform 5-10 sprints lasting no more than 15-30 seconds each, with full recovery between sprints (typically 3-4 times the sprint duration). Aim to do sprint workouts 2-3 times a week.
3. Cardiovascular Exercise: While resistance training takes center stage, cardiovascular exercise contributes significantly to overall health. Moderate-intensity cardio activities like jogging, cycling, jumping rope, rebounding, or sauna enhance cardiovascular well-being, complementing the body's holistic fitness.
4. Avoid Overtraining: Guarding against overtraining, characterized by excessive exercise without adequate recovery, is crucial for hormonal health. Prolonged intense workouts may elevate cortisol levels, a stress hormone with adverse effects on testosterone production. Adequate recovery time is essential to prevent the pitfalls of overtraining. Checking biometrics such as HRV is a great evidence-based indicator to quantify stress in the system.
A sample full-body workout three times a week might include:
- Warm-up
- 4 sets of 8 reps of bench press and squats
- 4 sets of 8 reps of deadlifts and pull-ups
- 6 sets of 10-second sprints
- Cool-down
A well-rounded exercise routine embraces resistance training (consisting of novel exercises to address specific musculoskeletal, biomechanical imbalances, and breathing mechanics), HIIT, and moderate-intensity cardio. Each type of exercise brings unique contributions to hormonal balance, offering comprehensive benefits for overall health. The body intricately adapts hormonally to the demands imposed during exercise. Thoughtfully designed, regular exercise routines can instigate positive hormonal adaptations, fostering improved testosterone regulation.
Acknowledging the diversity of individual responses to exercise is paramount. Tailoring routines to personal preferences and fitness levels ensures sustainable and enjoyable exercise habits, promoting long-term commitment. Consistency emerges as the linchpin for reaping long-term hormonal benefits from exercise. Establishing a regular routine that integrates various exercise types contributes not only to hormonal well-being but also to overall health.
In conclusion, the synergy of cardiovascular and resistance training exercises presents a potent strategy for optimizing testosterone levels. Resistance training, with a focus on compound movements and weightlifting, sparks testosterone production, while the inclusion of HIIT and cardiovascular exercise contributes holistically to health. Vigilance against overtraining and allowing sufficient recovery time are pivotal in maintaining hormonal balance. By adopting a balanced and personalized exercise routine, individuals can actively support hormonal health, enhancing their overall well-being.
Stress Management
Techniques that induce relaxation activate the parasympathetic nervous system (PNS), which counters the fight-or-flight response associated with chronic stress. By calming the SNS, they help restore hormonal balance, positively impacting testosterone levels. This increase in the PNS can directly improve sleep quality. Quality sleep is essential for optimal hormonal function, including testosterone production during the night.
As with many of the solutions addressed, there is trend in the underlying mechanisms that result in improved hormonal balance, including reduced inflammation, improved mood and mental health, which fosters a mind-body connection that allows individuals to better understand and manage stress triggers. By increasing self-awareness, individuals can make conscious choices that positively impact their hormonal responses, thereby facilitating changes in thought patterns and behaviors related to stress. A more adaptive response to stressors can reduce the physiological impact on hormones, including testosterone.
Stress management practices positively influence the communication between the brain and endocrine glands. Improved hormonal communication supports optimal functioning of the hypothalamus, pituitary gland, and testes, essential for testosterone regulation.
As with most practices that induce positive health effects, regular practice offers cumulative benefits over time. Consistency in these techniques contributes to sustained stress resilience and supports ongoing hormonal health. Anyone interested in optimizing hormone levels is encouraged to adopt a holistic perspective of well-being by addressing physical, mental, and emotional aspects of health. A balanced and integrated approach to well-being positively influences hormonal health, including testosterone levels.
As mentioned, incorporating meditation, yoga, or mindfulness, music, dancing, humor and laughter - whatever helps you relax - into one's routine can be a powerful strategy for managing chronic stress and supporting hormonal health. It's essential to choose techniques that resonate with individual preferences and consistently practice them to reap the long-term benefits.

Cold Water Immersion
Cold exposure activates the hypothalamus, a region of the brain that plays a central role in the regulation of hormonal balance. The hypothalamus controls the release of gonadotropin-releasing hormone (GnRH), which, in turn, stimulates the pituitary gland to release luteinizing hormone (LH). LH acts on the testes, promoting the synthesis and release of testosterone.
From a vascular perspective, cold water immersion may lead to vasoconstriction (narrowing of blood vessels) followed by vasodilation (widening of blood vessels) in response to rewarming. This cycle of vasoconstriction and vasodilation can enhance blood flow, potentially increasing perfusion to the testes. Improved blood flow to the testes may support optimal Leydig cell function, which is crucial for testosterone production.
Metabolically, exposure to cold activates brown adipose tissue (BAT), a type of fat tissue that generates heat. BAT activation is associated with increased energy expenditure and metabolic activity. Some studies suggest that BAT activation may positively influence hormonal regulation, including testosterone production.
Cold water immersion has also been demonstrated to have anti-inflammatory effects. Chronic inflammation is associated with disruptions in not only energy production, but hormonal balance as well, including reduced testosterone levels. By reducing inflammation, cold water immersion may support a more favorable hormonal environment.
Additionally, some individuals report improved sleep quality following cold water immersion, likely due to enhanced thermoregulation. Quality sleep is crucial for overall health, including hormonal regulation. Improved sleep may indirectly contribute to optimal testosterone levels.
Cold exposure is considered a form of hormetic stress, stimulating cold shock proteins a mild stressor that, when applied in moderation, may lead to adaptive responses. Hormetic stressors, such as cold exposure, have been proposed to stimulate the body's adaptive mechanisms, including the endocrine system, potentially leading to increased testosterone production.
From a neurobiological perspective, cold water immersion has been associated with an increase in neurotransmitters, such as dopamine and norepinephrine levels, which can beneficially impact mood and behavior. However, what is not often described by proponents of cold water immersion is that exposure to extreme cold (determined by the individual's physiology, experience and perception) can trigger a cascade of physiological responses leading to elevated adrenaline (epinephrine) levels, thereby causing excess stress, and moving the needle in the opposite direction, away from optimal hormonal balance. Cold water immersion can certainly activate the sympathetic nervous system, otherwise known as the body's "fight or flight" response, which is why it is best to perform cold water immersion sometime in the morning or mid-day.
Dopamine and norepinephrine are neurotransmitters that play key roles in the regulation of mood, attention, and arousal. Cold water immersion has been shown to stimulate the release of both dopamine and norepinephrine in response to the stress of cold exposure. Dopamine has been suggested to have a regulatory role in the release of adrenaline. Studies indicate that dopamine, acting through specific receptors, may influence the release of adrenaline from the adrenal medulla. The overall response to cold stress involves the activation of the hypothalamus-pituitary-adrenal (HPA) axis, leading to the release of stress hormones, including cortisol and adrenaline.
It is important to realize that just because some is good, more is not always better. Deliberate cold exposure can absolutely improve quality of life, via hormonal mechanisms. However, if performed improperly, cold water immersion can disrupt hormonal effects. It is ideal to start low and slow. In other words, use a low dose and progressively increase as tolerance improves. As always, listen to your body. Shivering is a sign that the body is too cold.
Regulating Ejaculation Frequency
Recommended Ejaculation Frequency by Age:
- 20s: As desired
- 30s: 3-4 times per week
- 40s: 2-3 times per week
- 50s: 1-2 times per week
- 60+: Once a week, depending on health
A popular method among Tao practitioners is to ejaculate only two or three times out of every ten sexual encounters. Sun Simiao, a notable Tao theorist, advises men over 50 to ejaculate no more than once every 20 days, and men over 60 no more than once every 100 days.
To practice "injaculation" rather than ejaculation, men can squeeze the muscles used to stop urine flow while "breathing the energy" up the spine. If ejaculation seems imminent, applying pressure to the perineum (the area between the scrotum and anus) can help delay it. Elaboration of this technique can be found in the book, The Multiorgasmic Man.
This technique is cost-free but may require some patience to avoid becoming moody or aggressive due to sexual frustration. On the other hand, researchers at Boston University School of Public Health found that ejaculating at least 21 times a month may reduce the risk of prostate cancer.
Other Lifestyle Strategies
- Adequate Sleep: Ensure sufficient and quality sleep. Sleep plays a crucial role in hormonal regulation, including testosterone production. Aim for 7-9 hours of sleep per night. This also include mitigating non-native EMF exposure via blue lights in the environment. Blue light exposure is well documented to disrupt circadian rhythms, thereby impairing recovery mechanisms, and disrupting the delicate balance of hormones.
- Maintain a Healthy Weight: Achieve and maintain a healthy weight. Obesity is associated with lower testosterone levels, and losing excess weight can positively impact hormonal balance. A general rule of thumb is a healthy BMI, although for individuals who have large amounts of muscle, the reliability of BMI falls short.
- Vitamin D: Ensure adequate vitamin D levels. Vitamin D deficiency has been linked to lower testosterone levels, among many other comorbidities and increased all-cause mortality. It is ideal to spend time in sunlight at solar noon, and avoid vitamin D supplements. Our ancient ancestors spent nearly all of their time outside, and did not have access to synthetic products.
- Limit Alcohol Consumption: Moderate alcohol intake, as excessive alcohol consumption has been associated with lower testosterone levels. To date, there are zero benefits of alcohol consumption, perhaps (although loosely) with exception to social connection - of course, there are limits to the risk:benefit ratio with respect to the amount of alcohol consumed. Alcohol is a known toxin directly connected to a variety of comorbidities.
Navigating andropause involves a multifaceted approach that addresses both hormonal changes and lifestyle factors. By incorporating these science-backed nutrition and lifestyle strategies, men can optimize their well-being during this natural phase of life. Always consult with a healthcare professional for personalized advice based on individual health needs.
Optimizing Your Testosterone: A Day in the Life
Start your day with getting sun into your eyes. Afterwards, consume a breakfast rich in healthy fats like avocado, eggs, grass-fed butter and cheese, oyster mushrooms, sauerkraut, and coconut milk. Along with breakfast, take the following supplements:
- 5g creatine
- 50mg supplemental DHEA
- 10mg boron
- 30g cocoa powder
- 2g maca root extract
- 250 mg shilajit
- 500mg fenugreek extract
- 200mg Pycnogenol
- 200-300mg Eurycoma longifolia
- 300mg Tribulus
Throughout the Day:
Be sure to get sun on your skin at solar noon in an effort to create vitamin D. Implement EMF mitigation strategies by keeping your phone in airplane mode when not in use, avoiding using your laptop on your lap (or using an anti-radiation devices, such as Aires Tech), and turning off Wi-Fi on devices when using ethernet. Detox your home and consider auditing your workspace for EMF with an acoustimeter or by hiring a building biologist. Manage stress to maintain a high testosterone ratio. Practice relaxation techniques like deep nasal and belly breathing, laugh, smile, get outdoors, and be mindful of stress mitigation strategies. Spend time with people, especially women, as their presence can boost testosterone levels. Avoid pornography as it can negatively impact hormonal balance.
Afternoon Workout:
For an effective testosterone-boosting workout, do the following exercises with heavy weights, ensuring good form. Perform 5 sets of 5 reps each:
- Bench Press
- Deadlift
- Front Squat
- Shoulder Press
- Clean
Evening:
Enhance carbon dioxide levels to augment the efficiency of oxygen transport and energy production.
Integral Wellness Program: All in one Approach
1. Evidence-Based & Holistic Approach:
- Rooted in evidence-based practices, the Integral Wellness Program prioritizes approaches backed by scientific research.
- This credible and reliable program adopts a holistic model, recognizing the interconnectedness of physical, mental, and emotional well-being.
- By addressing multiple dimensions of health, it aims to create a synergistic effect, optimizing the conditions for hormonal balance.
- The program unfolds in a step-by-step manner, offering clear protocols for implementation.
- This structured approach simplifies the journey, making it accessible for individuals seeking a systematic and manageable process.
- Recognizing that each individual is unique, the program provides personalized guidance tailored to specific needs and goals.
- Customization ensures that the approach resonates with individual preferences and aligns with their health objectives.
- Integral to the program are nutritional strategies designed to support hormone optimization.
- These strategies likely include guidance on nutrient-dense foods, dietary patterns, and specific nutrients beneficial for hormonal health.
- Beyond nutrition, the program delves into lifestyle optimization.
- Factors such as sleep, stress management, and physical activity are likely addressed, recognizing their significant impact on hormonal balance.
- This holistic approach acknowledges the influence of mental well-being on hormonal health.
- The program likely provides educational resources, empowering individuals with knowledge about testosterone, hormonal health, and the impact of lifestyle choices.
- Informed decisions are pivotal to sustained well-being.
- The program is available through an accessible online platform, enabling participants to engage at their own pace and convenience.
- Flexibility in participation facilitates seamless integration into daily life.
In essence, the Integral Wellness Program serves as a comprehensive guide for those embarking on a journey to optimize testosterone levels. Through its holistic and step-by-step approach, individuals can navigate the intricacies of well-being, unlocking the potential for sustained hormonal health.
Movement | nutrition | lifestyle |
References
Magnesium deficiency is often misdiagnosed because it does not show up in blood tests - only 1% of the body's magnesium is stored in the blood
| Most doctors and laboratories don't even include magnesium status in routine blood tests. Thus, most doctors don't know when their patients are deficient in magnesium, even though studies show that 80% of Americans are deficient in magnesium. Magnesium is essential for hundreds (300-600) of metabolic function, including but not limited to:
|
One has to recognize the signs of magnesium thirst or hunger on their own since allopathic medicine is lost in this regard. It is really something much more subtle then hunger or thirst but it is comparable. In a world though where doctors and patients alike do not even pay attention to thirst and important issues of hydration, it is not hopeful that we will find many recognizing and paying attention to magnesium thirst and hunger, which is a dramatic way of expressing the concept of magnesium deficiency.
Few people are aware of the enormous role magnesium plays in our bodies. Magnesium is by far the most important mineral in the body. After oxygen, water, and basic food, magnesium may be the most important element needed by our bodies; vitally important, yet hardly known. It is more important than calcium, potassium or sodium and regulates all three of them. Millions suffer daily from magnesium deficiency without even knowing it.
In fact, there happens to be a relationship between what we perceive as thirst and deficiencies in electrolytes. I remember a person asking, "Why am I dehydrated and thirsty when I drink so much water?" Thirst can mean not only lack of water but it can also mean that one is not getting enough nutrients and electrolytes. Magnesium, Potassium, Bicarbonate, Chloride and Sodium are some principle examples and that is one of the reasons magnesium chloride is so useful.
You know all those years, when doctors used to tell their patients 'its all in your heads,' were years the medical profession was showing its ignorance. It is a torment to be magnesium deficient on one level or another. Even if it's for the enthusiastic sport person whose athletic performance is down, magnesium deficiency will disturb sleep and background stress levels and a host of other things that reflect on the quality of life. Doctors have not been using the appropriate test for magnesium - their serum blood tests just distort their perceptions. Magnesium has been off their radar screens through the decades that magnesium deficiencies have snowballed.
Symptoms of Magnesium Deficiency
A full outline of magnesium deficiency was beautifully presented in a recent article by Dr. Sidney Baker. "Magnesium deficiency can affect virtually every organ system of the body. With regard to skeletal muscle, one may experience twitches, cramps, muscle tension, muscle soreness, including back aches, neck pain, tension headaches and jaw joint (or TMJ) dysfunction. Also, one may experience chest tightness or a peculiar sensation that he can't take a deep breath. Sometimes a person may sigh a lot."
"Symptoms involving impaired contraction of smooth muscles include constipation; urinary spasms; menstrual cramps; difficulty swallowing or a lump in the throat-especially provoked by eating sugar; photophobia, especially difficulty adjusting to oncoming bright headlights in the absence of eye disease; and loud noise sensitivity from stapedius muscle tension in the ear."
"Other symptoms and signs of magnesium deficiency and discuss laboratory testing for this common condition. Continuing with the symptoms of magnesium deficiency, the central nervous system is markedly affected. Symptoms include insomnia, anxiety, hyperactivity and restlessness with constant movement, panic attacks, agoraphobia, and premenstrual irritability. Magnesium deficiency symptoms involving the peripheral nervous system include numbness, tingling, and other abnormal sensations, such as zips, zaps and vibratory sensations."
"Symptoms or signs of the cardiovascular system include palpitations, heart arrhythmias, and angina due to spasms of the coronary arteries, high blood pressure and mitral valve prolapse. Be aware that not all of the symptoms need to be present to presume magnesium deficiency; but, many of them often occur together. For example, people with mitral valve prolapse frequently have palpitations, anxiety, panic attacks and premenstrual symptoms. People with magnesium deficiency often seem to be "uptight." Other general symptoms include a salt craving, both carbohydrate craving and carbohydrate intolerance, especially of chocolate, and breast tenderness."
Magnesium is needed by every cell in the body including those of the brain. It is one of the most important minerals when considering supplementation because of its vital role in hundreds of enzyme systems and functions related to reactions in cell metabolism, as well as being essential for the synthesis of proteins, for the utilization of fats and carbohydrates. Magnesium is needed not only for the production of specific detoxification enzymes but is also important for energy production related to cell detoxification. A magnesium deficiency can affect virtually every system of the body.
One of the principle reason doctors write millions of prescriptions for tranquilizers each year is the nervousness, irritability, and jitters largely brought on by inadequate diets lacking magnesium. Persons only slightly deficient in magnesium become irritable, highly-strung, and sensitive to noise, hyper-excitable, apprehensive and belligerent. If the deficiency is more severe or prolonged, they may develop twitching, tremors, irregular pulse, insomnia, muscle weakness, jerkiness and leg and foot cramps.
If magnesium is severely deficient, the brain is particularly affected. Clouded thinking, confusion, disorientation, marked depression and even the terrifying hallucinations of delirium tremens are largely brought on by a lack of this nutrient and remedied when magnesium is given. Because large amounts of calcium are lost in the urine when magnesium is under supplied, the lack of this nutrient indirectly becomes responsible for much rampant tooth decay, poor bone development, osteoporosis and slow healing of broken bones and fractures. With vitamin B6 (pyridoxine), magnesium helps to reduce and dissolve calcium phosphate kidney stones.
Magnesium deficiency may be a common factor associated with insulin resistance. Symptoms of MS that are also symptoms of magnesium deficiency include muscle spasms, weakness, twitching, muscle atrophy, an inability to control the bladder, nystagmus (rapid eye movements), hearing loss, and osteoporosis. People with MS have higher rates of epilepsy than controls. Epilepsy has also been linked to magnesium deficiencies.[1]
Gastrointestinal UPSET with Magnesium
The nature of magnesium salts is the resulting osmotic effect - the potential laxative effect is due to magnesium (salts) remaining in the intestines causing water to be drawn toward the salts for equilibrium, leading to gastrointestinal distress. While it is often recommended to take magnesium with food, some potential methods to offset the laxative effect would be to consume it fasted, augmenting absorption, and/or improve hydration by consuming more water, ideally prepared by reverse osmosis.
Another way you can avoid that laxative effect is stop the consumption of magnesium citrate and taking molecular hydrogen, which contains pure ionic metallic magnesium.
early warning symptoms suggestive of magnesium insufficiency
- Physical and mental fatigue
- Persistent under-eye twitch
- Tension in the upper back, shoulders and neck
- Headaches
- Pre-menstrual fluid retention and/or breast tenderness
Possible manifestations of magnesium deficiency include
We hear all the time about how heart disease is the number one health crisis in the country, about how high blood pressure is the "silent killer", and about how ever increasing numbers of our citizens are having their lives and the lives of their families destroyed by diabetes, Alzheimer's disease, and a host of other chronic diseases.
Signs of severe magnesium deficiency include
- Extreme thirst
- Extreme hunger
- Frequent urination
- Sores or bruises that heal slowly
- Dry, itchy skin
- Unexplained weight loss
- Blurry vision that changes from day to day
- Unusual tiredness or drowsiness
- Tingling or numbness in the hands or feet
- Frequent or recurring skin, gum, bladder or vaginal yeast infections
Magnesium deficiency is synonymous with diabetes and is at the root of many if not all cardiovascular problems.
Magnesium deficiency is a predictor of diabetes and heart disease both; diabetics both need more magnesium and lose more magnesium than most people. In two new studies, in both men and women, those who consumed the most magnesium in their diet were least likely to develop type 2 diabetes, according to a report in the January 2006 issue of the journal Diabetes Care. Until now, very few large studies have directly examined the long-term effects of dietary magnesium on diabetes. Dr. Simin Liu of the Harvard Medical School and School of Public Health in Boston says, "Our studies provided some direct evidence that greater intake of dietary magnesium may have a long-term protective effect on lowering risk," said Liu, who was involved in both studies.
The thirst of diabetes is part of the body's response to excessive urination. The excessive urination is the body's attempt to get rid of the extra glucose in the blood. This excessive urination causes the increased thirst. But we have to look at what is causing this level of disharmony. We have to probe deeper into layers of cause. The body needs to dump glucose because of increasing insulin resistance and that resistance is being fueled directly by magnesium deficiency, which makes toxic insults more damaging to the tissues at the same time.
When diabetics get too high blood sugars, the body creates "ketones" as a by-product of breaking down fats. These ketones cause blood acidity which causes "acidosis" of the blood, leading to Diabetic Ketoacidosis (DKA), This is a very dangerous condition that can lead to coma and death. It is also called "diabetic acidosis", "ketosis", "ketoacidosis" or "diabetic coma". DKA is a common way for new Type 1 diabetics to be diagnosed. If they fail to seek medical advice on symptoms like urination, which is driving thirst they can die of DKA.
Oral magnesium supplements reduce erythrocyte[2] dehydration.[3] In general, optimal balances of electrolytes are necessary to maintain the best possible hydration. Diabetic thirst is initiated specifically by magnesium deficiency with relative calcium excess in the cells. Even water, our most basic nutrient starts having a hard time getting into the cells with more going out through the kidneys.
Autism and Magnesium Deficiency
1) The foods they are eating are stripped of magnesium because foods in general, as we shall see below are declining in mineral content in an alarming way.
2) The foods many children eat are highly processed junk foods that do not provide real nutrition to the body.
3) Because most children on the spectrum are not absorbing the minerals they need even when present in the gut. Magnesium absorption is dependent on intestinal health, which is compromised totally in leaky gut syndromes and other intestinal problems that the majority of autism syndrome disorders.
4) Because the oral supplements doctors rely on are not easily absorbed, because they are not in the right form and because magnesium in general is not administered easily orally.
Modern medicine is supposed to help people not hurt them, but with their almost total ignorance of magnesium doctors end up hurting more than they help for many of the medical interventions drive down magnesium levels when they should be driving them up. Many if not most pharmaceutical drugs drive magnesium levels into very dangerous zones and surgery done without increasing magnesium levels is much more dangerous then surgery done with.
The foundation of medical arrogance is actually medical ignorance and the only reason ignorance and arrogance rule the playing field of medicine is a greed lust for power and money. Human nature seems to be at its worst in modern medicine when it should be at its best. It is sad that people have to suffer needlessly and extraordinarily tragic that allopathic medicine has turned its back on the Hippocratic Oath and all that it means.
Resources
[2] Red blood cells are also known as RBCs, red blood corpuscles (an archaic term), haematids or erythrocytes (from Greek erythros for "red" and kytos for "hollow", with cyte translated as "cell" in modern usage). The capitalized term Red Blood Cells is the proper name in the US for erythrocytes in storage solution used in transfusion medicine.
[3] J. Clin. Invest. 100(7): 1847-1852 (1997). doi:10.1172/JCI119713. The American Society for Clinical Investigation
Guest: Dr. Richard Urso
Guests: Jonathan Isaac, Aaron Kheriaty M.D., Aaron Siri, Esq.
Guests: Dr. Mark McDonald, AJ DePriest
| This systematic review and meta-analysis are designed to determine whether there is empirical evidence to support the belief that “lockdowns” reduce COVID-19 mortality. Lockdowns are defined as the imposition of at least one compulsory, non-pharmaceutical intervention (NPI). NPIs are any government mandate that directly restrict peoples’ possibilities, such as policies that limit internal movement, close schools and businesses, mandated face masks, and ban international travel. This study employed a systematic search and screening procedure in which 18,590 studies are identified that could potentially address the belief posed. After three levels of screening, 34 studies ultimately qualified. |
While this meta-analysis concludes that lockdowns have had little to no public health effects, they have imposed enormous economic and social costs where they have been adopted. In consequence, lockdown policies are ill-founded and should be rejected as a pandemic policy instrument.
| a_literature_review_and_meta-analysis_of_the_effects_of_lockdowns_on_covid-19_mortality.pdf | |
| File Size: | 2332 kb |
| File Type: | |
GUESTS: Fireman John Knox, Prof. Norman Fenton, Gina Doane, Deb Conrad, PA-C
Guests: Aaron Siri, Esq., Geert Vanden Bossche, PhD, DVM
Archives
May 2024
April 2024
March 2024
February 2024
January 2024
December 2023
September 2023
August 2023
June 2023
October 2022
July 2022
February 2022
January 2022
December 2021
November 2021
October 2021
August 2021
July 2021
June 2021
March 2021
February 2021
January 2021
December 2020
November 2020
September 2020
August 2020
March 2020
December 2019
November 2019
October 2019
September 2019
August 2019
July 2019
May 2019
April 2019
March 2019
February 2019
January 2019
December 2018
November 2018
October 2018
September 2018
August 2018
July 2018
June 2018
April 2018
March 2018
February 2018
January 2018
December 2017
November 2017
October 2017
September 2017
August 2017
July 2017
June 2017
May 2017
April 2017
March 2017
February 2017
January 2017
Categories
All
5G
Adductors
Anxiety
Autism
Ayurveda
Big Pharma
Body
Breathing
Cancer
Cannabis
Carbohydrates
Cardiovascular Disease
Children
Chronic Disease
Cognition
Consciousness
Coronavirus
Covid
Cryotherapy
Depression
Deuterium
Diet
Dietary Guidelines
EMFs
Emotions
Endocrine Disruptors
Environment
Exercise
Farming
Fasting
Fats
Fluoride
Food
Food Like Product
Food-like Product
Forest
Gardening
Genetics
Glyphosate
GMOs
Hamstrings
Healing
Health
Herbalism
Hormones
HRV
Immunity
Infertility
Laughter
Lockdowns
Loneliness
Longevity
Masks
Meditation
Metabolism
Microbiome
Microwave Radiation
Mind
Mortality
Musculoskeletal System
Nature
Neuroplasticity
Nutrition
Omega 3
Omega-3
Organic
Pelvis/Thigh
Pesticides
Physical Therapy
Placebo
Pollution
Positive
Pregnancy
Prevention
Processed Foods
Psi
Quadriceps
Research
Retirement
Salt
Sleep
Spine/Thorax
Spirit
Stress
Sugar
Technology
Touch Screens
Toxicity
Vaccines
Well Being
Well-Being





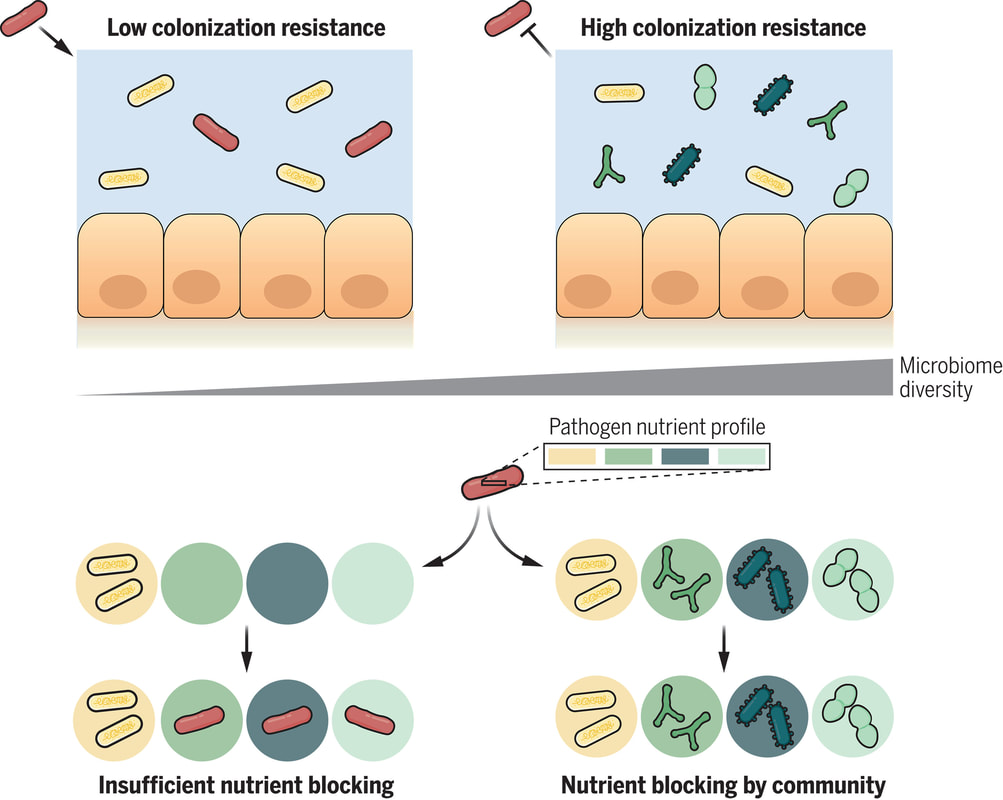
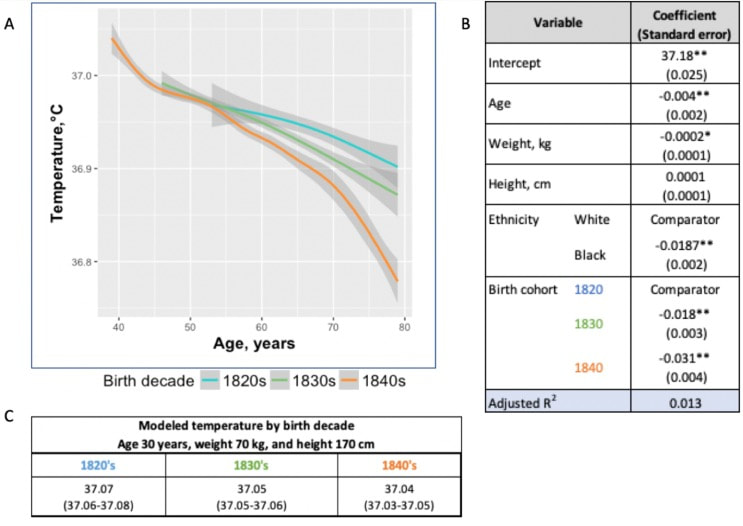
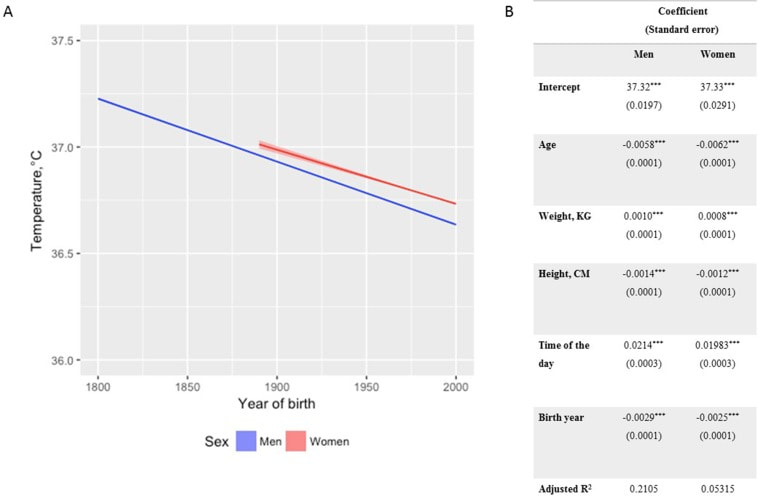

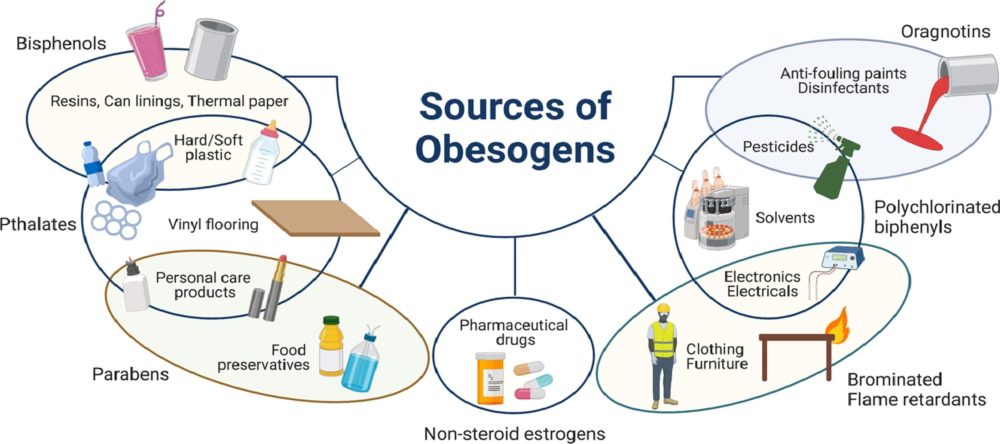

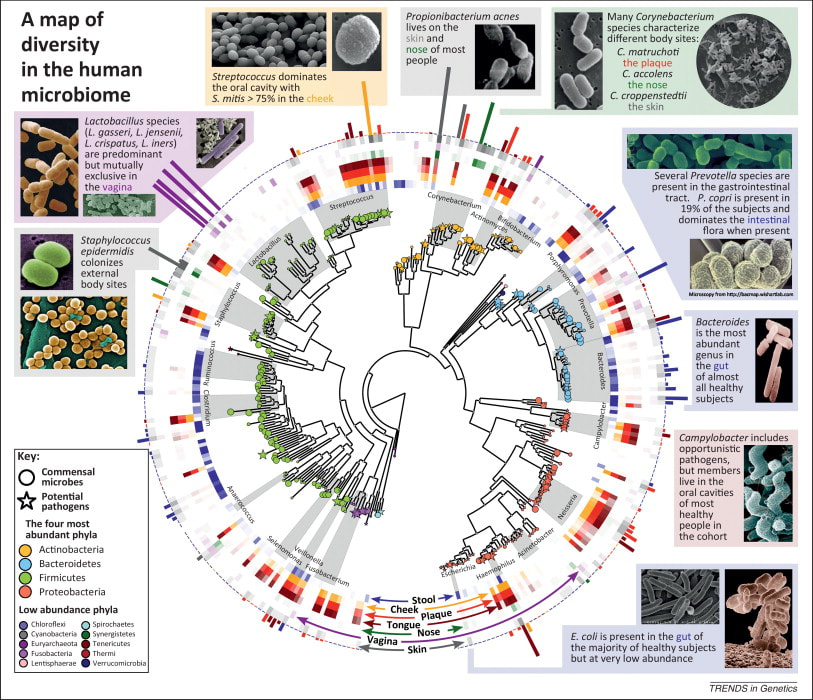
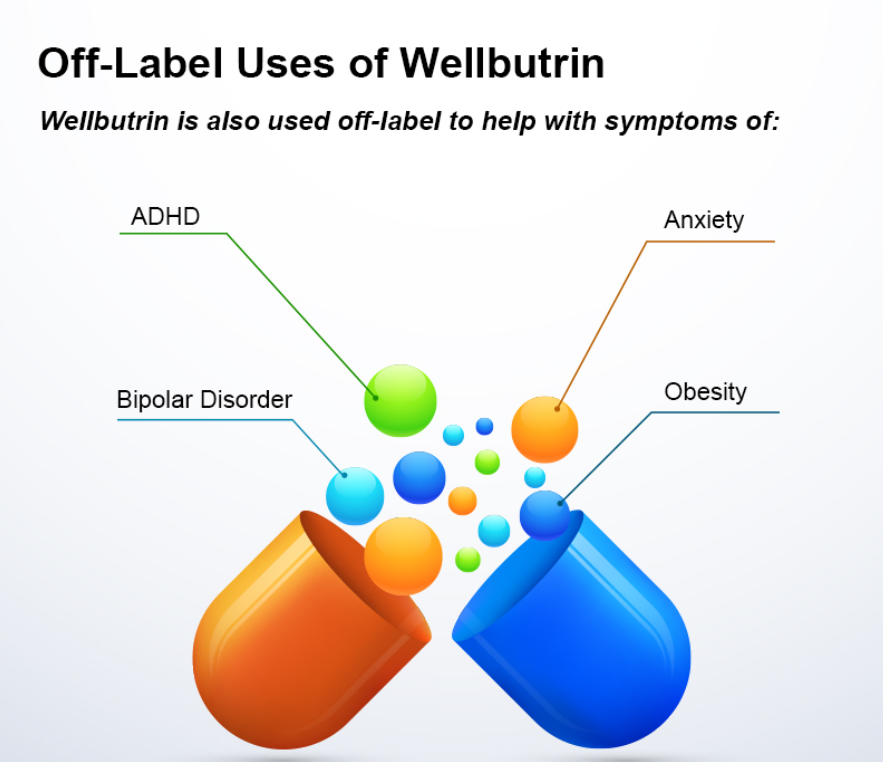
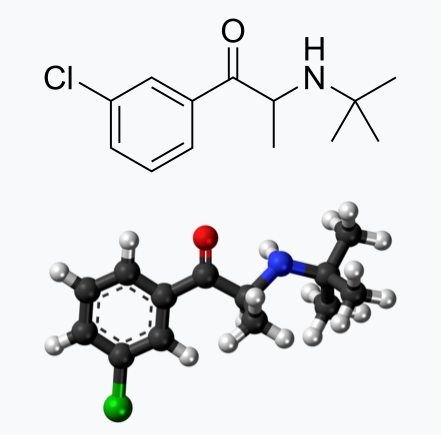

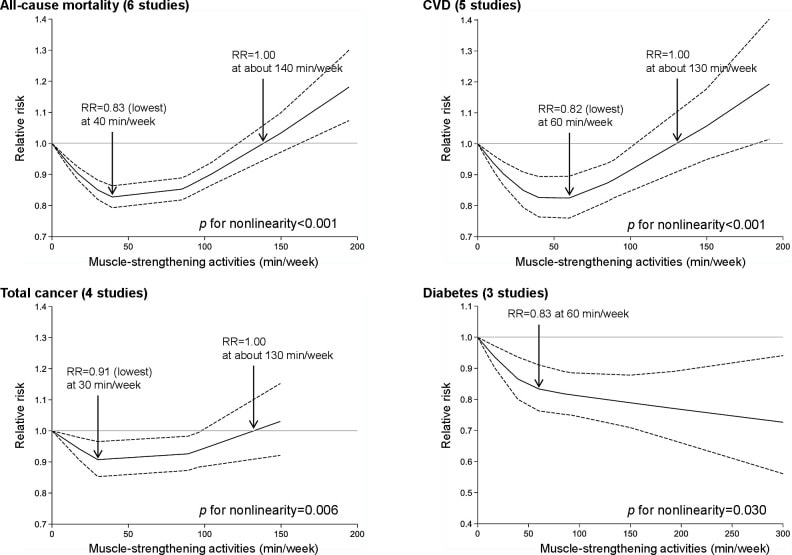
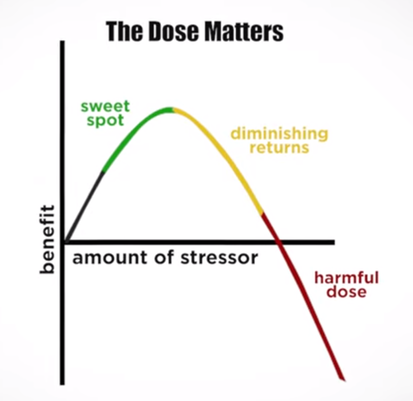
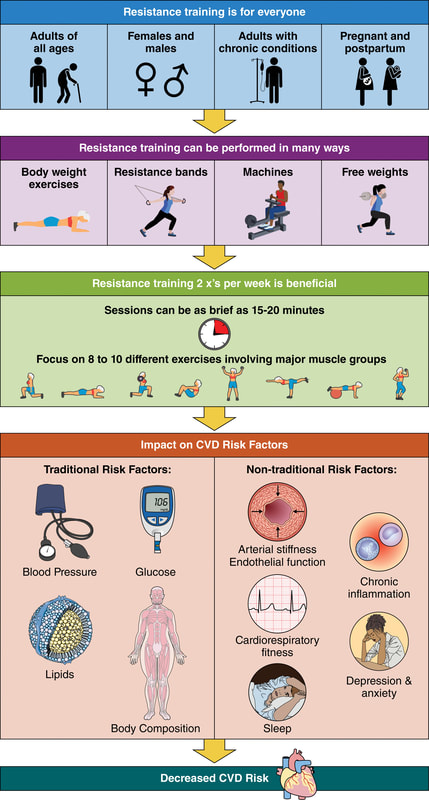
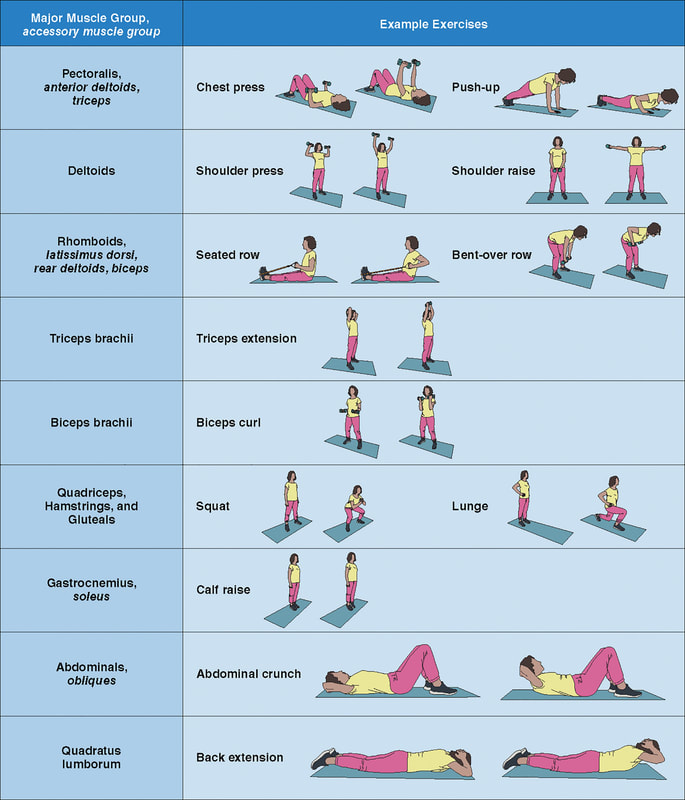
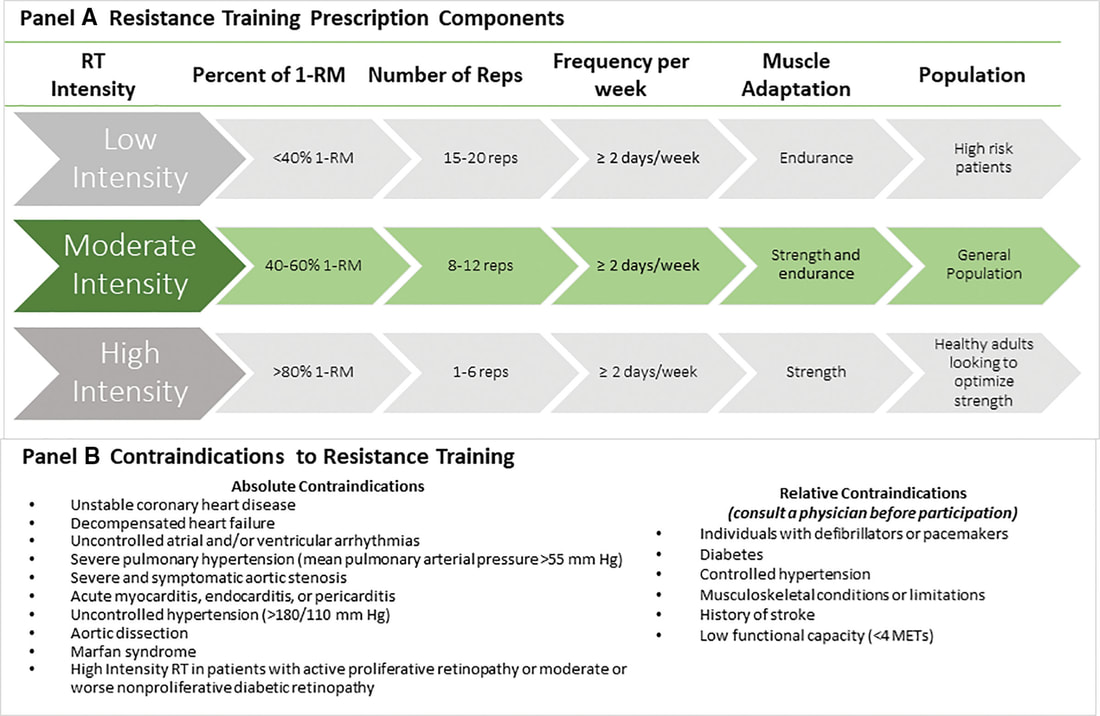
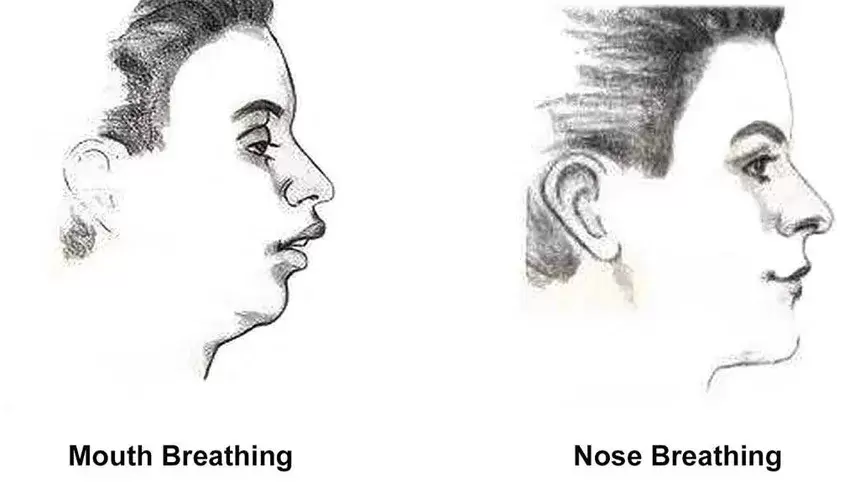
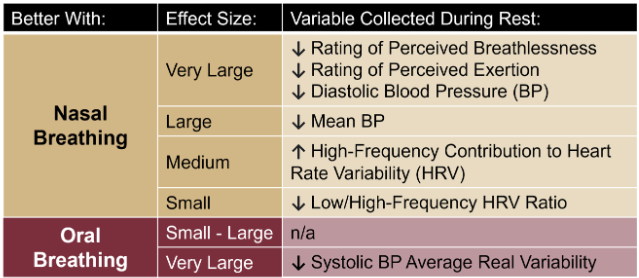
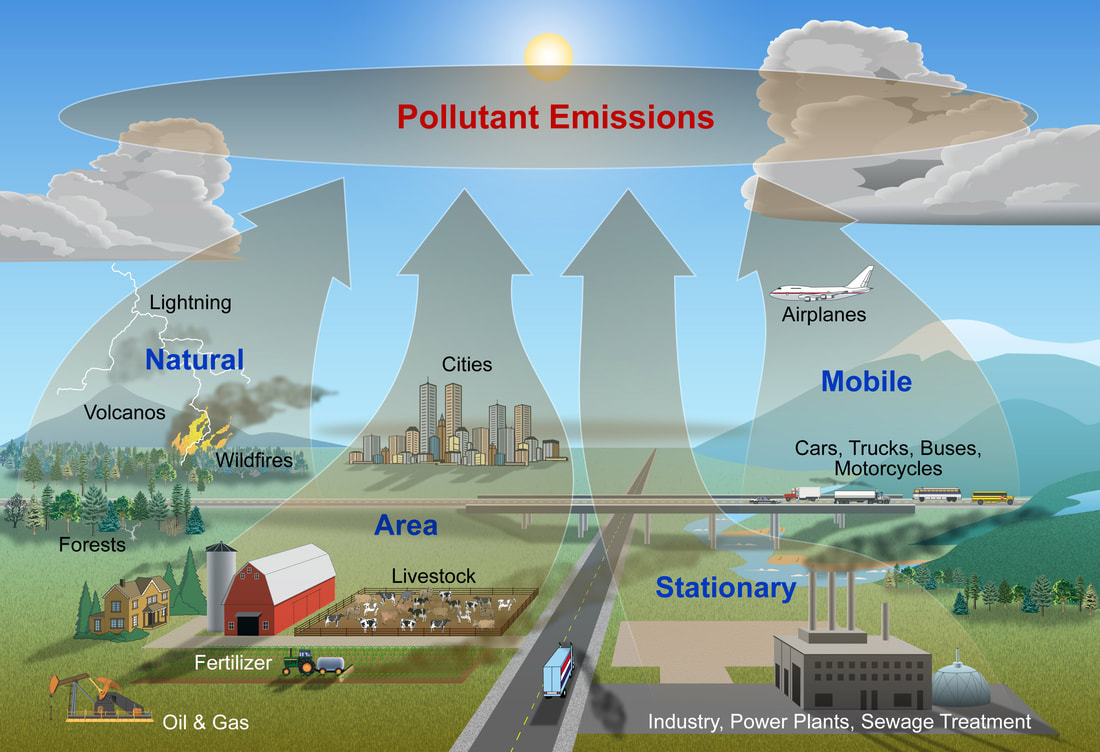
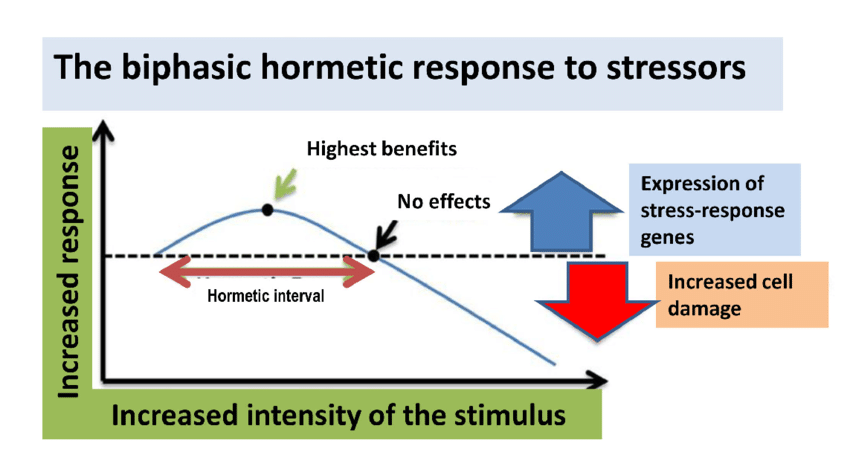
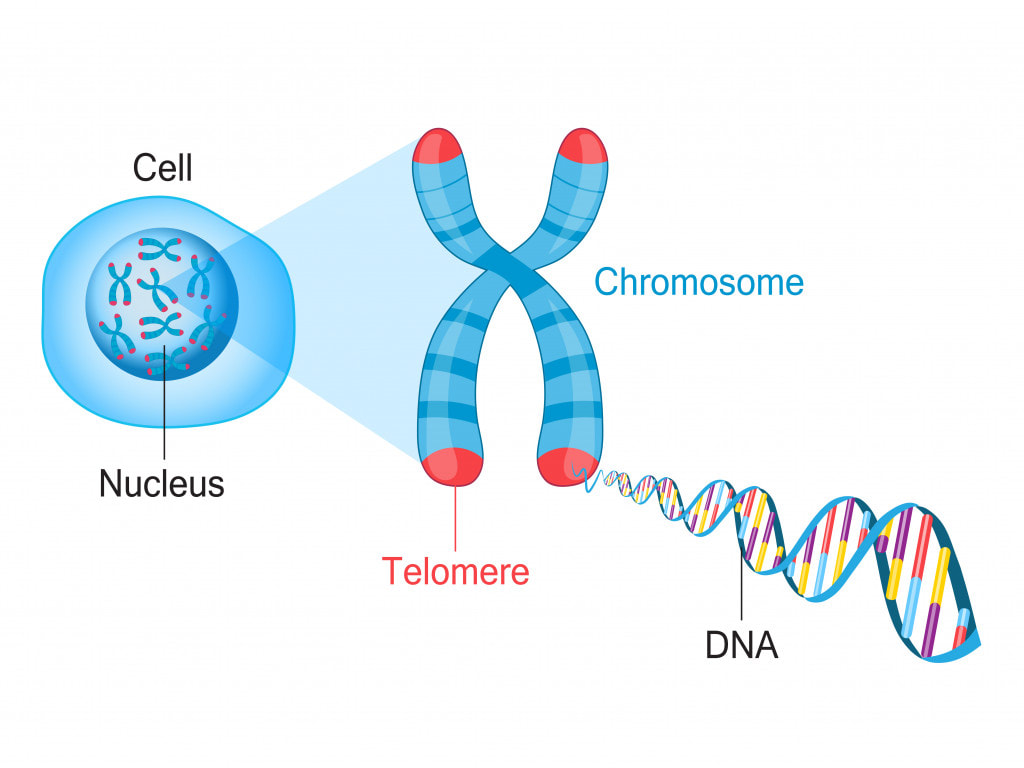

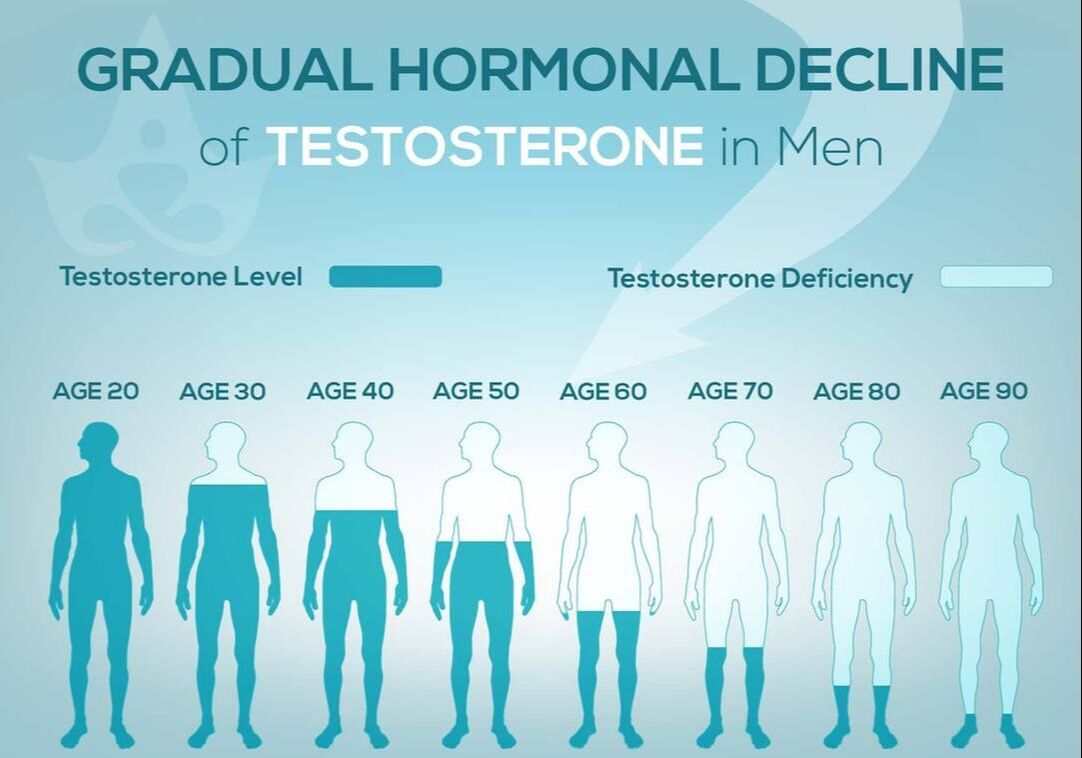

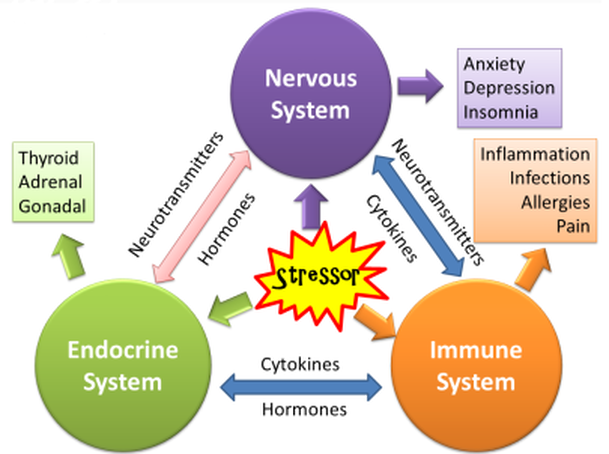
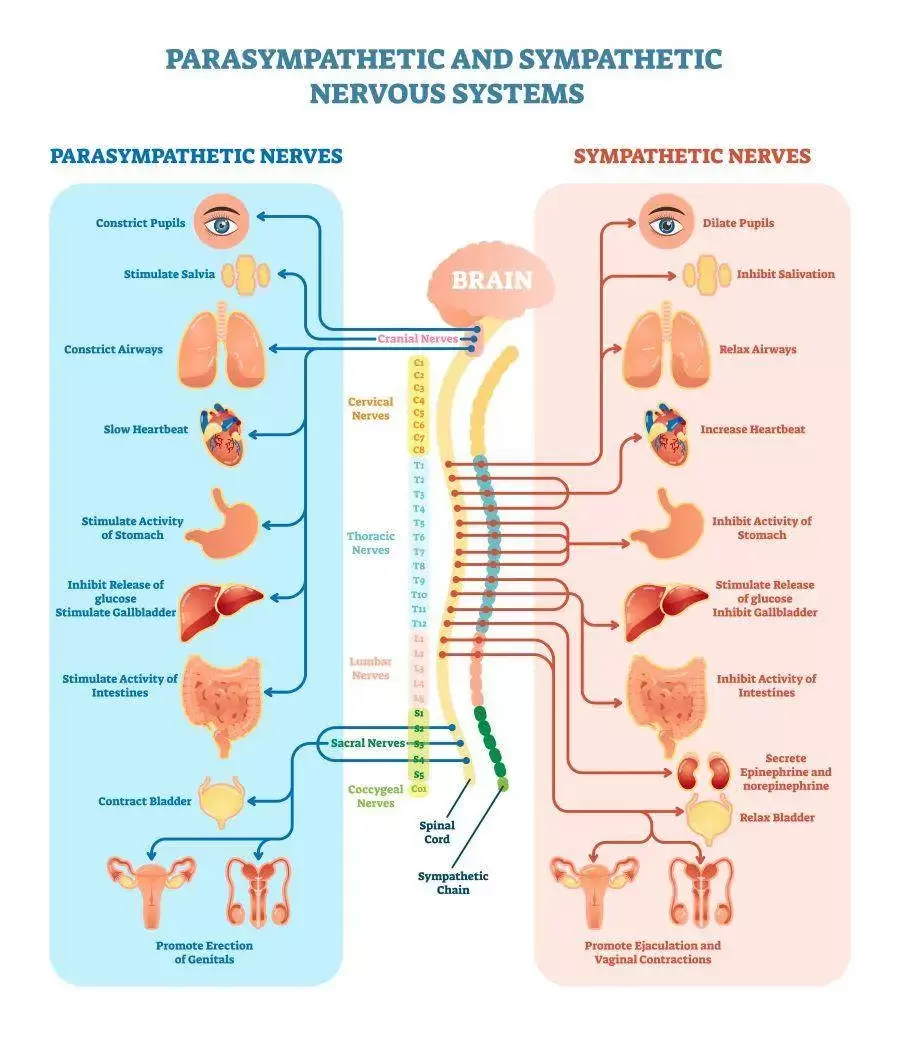
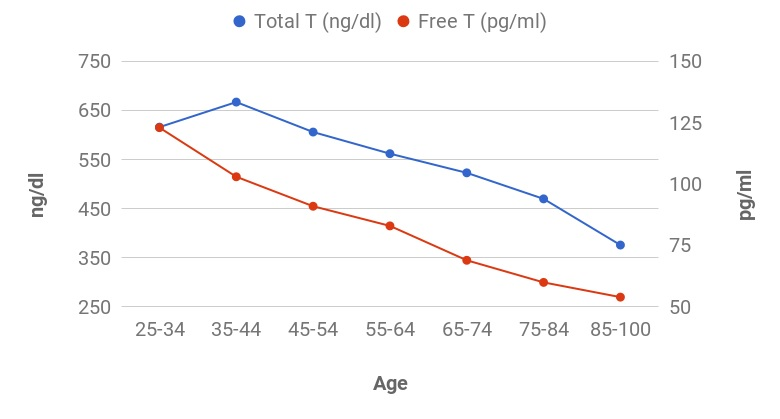
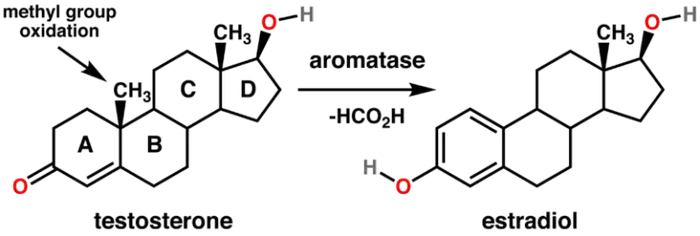
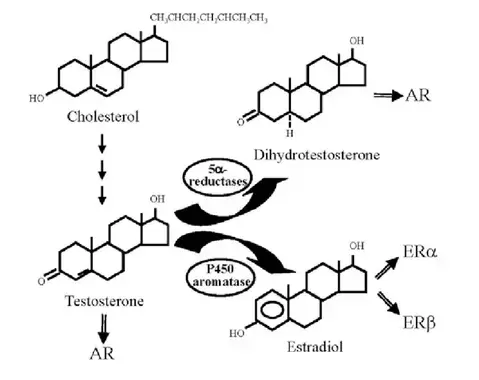



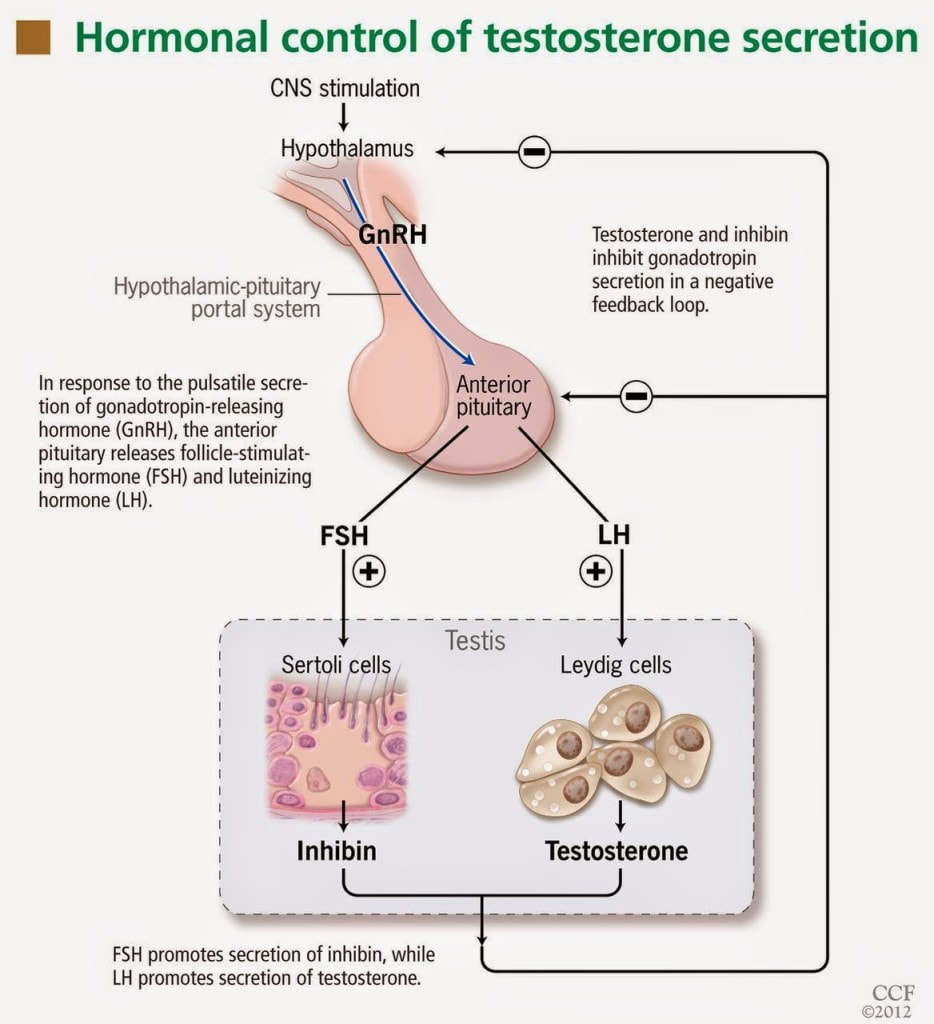



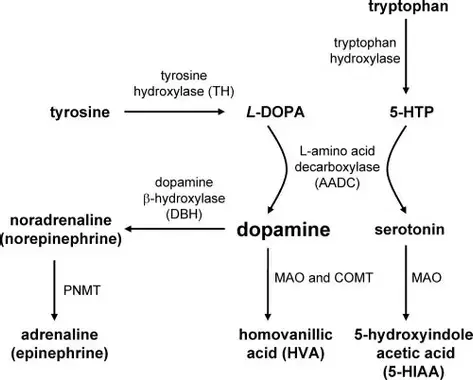





 RSS Feed
RSS Feed

