|
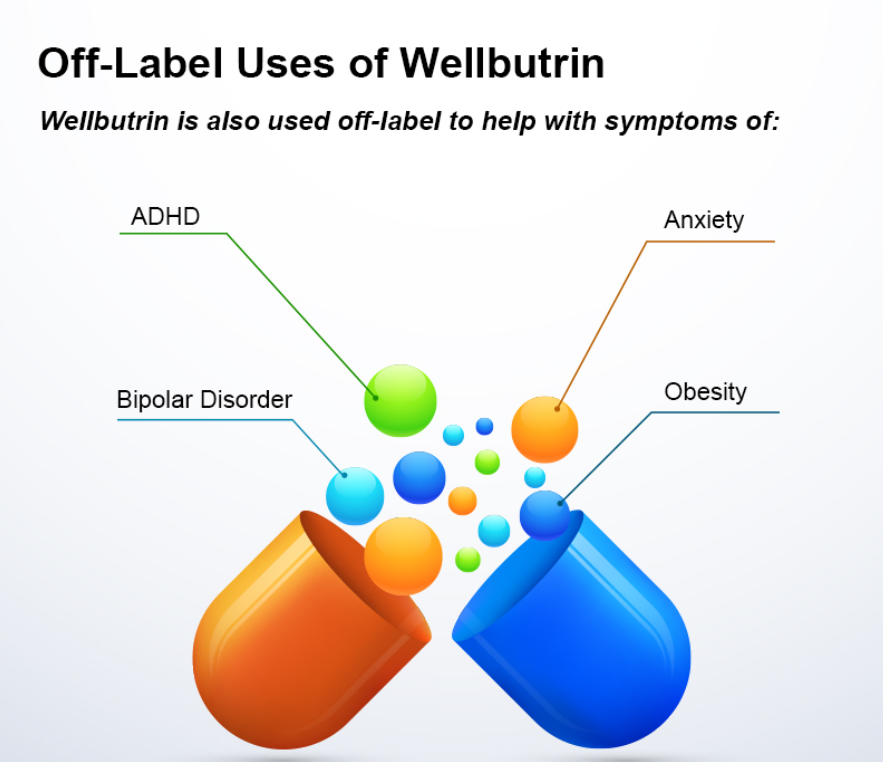
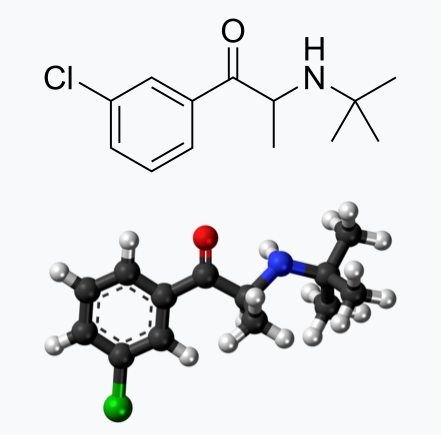
Bupropion, originally named Amfebutamone, sold under the brand name Wellbutrin, is a medication commonly prescribed for the treatment of major depressive disorder (MDD), as is often used off-label for attention deficit hyperactivity disorder (ADHD), anxiety, obesity, and bipolar disorder. While it has demonstrated efficacy in addressing certain mental health issues, it is essential to examine the potential harms associated with its use, particularly considering its modulation of neurotransmitters like norepinephrine (NE) and dopamine. This article aims to shed light on the risks of Wellbutrin use, with a focus on its implications for pregnant or lactating women. Additionally, we'll explore the idea that the indications for Wellbutrin may stem from underlying nutrition and lifestyle factors rather than a deficiency of the medication. prescription trendsAs of 2021, Bupropion, maintained its position as the 18th most prescribed drug in the United States. With an estimated 29,099,445 prescriptions filled, it remains a widely utilized medication in the realm of psychiatric pharmaceuticals. This notable figure underscores the prevalence of its use in addressing various conditions, including depression and smoking cessation. The estimated number of patients in the United States receiving Bupropion in 2021 reached 6,412,363. This statistic reflects the significant impact and reach of Bupropion across diverse patient populations. Its popularity could be attributed to its purported effectiveness in managing depressive disorders, ADHD, anxiety, and aiding individuals in smoking cessation efforts. descriptionWellbutrin (bupropion hydrochloride), unlike any other antidepressant on the market, is chemically characterized as a monocyclic aminoketone, is chemically unrelated to tricyclic, tetracyclic, selective serotonin re‑uptake inhibitor, or other known antidepressant agents. Its structure closely resembles that of diethylpropion; it is related to phenylethylamines. It is designated as (±)-1-(3-chlorophenyl)-2-[(1,1-dimethylethyl)amino]-propanone hydrochloride. Bupropion hydrochloride powder is white, crystalline, and highly soluble in water. It has a bitter taste and produces the sensation of local anesthesia on the oral mucosa. There is documented interindividual variability - everyone responds differently. Neurotransmitter Modulation: The Double-Edged SwordWellbutrin functions by influencing the levels of neurotransmitters in the brain, primarily inhibiting the breakdown of norepinephrine and dopamine. While this mechanism contributes to its antidepressant effects, it also raises concerns about potential side effects and risks. Altering neurotransmitter levels can lead to a range of adverse effects, with the most common including:
Here are some of the possible harms and common side effects associated with Wellbutrin:
While the approach of inhibiting the reuptake of dopamine and norepinephrine aims to enhance the availability of these neurotransmitters in the brain, an imbalance can lead to adverse effects. Excessive levels or prolonged elevated concentrations of these neurotransmitters may contribute to overstimulation and disrupt normal neural signaling. Norepinephrine is a neurotransmitter involved in the body's "fight or flight" response, influencing heart rate and blood pressure. Medications that impact norepinephrine reuptake can lead to cardiovascular side effects, including increased heart rate and elevated blood pressure. Individuals with pre-existing cardiovascular conditions may be at a higher risk for complications. Altered levels of dopamine and norepinephrine can influence mood and behavior. In some cases, inhibiting reuptake may contribute to psychiatric symptoms such as anxiety, restlessness, or irritability. Balancing the desired therapeutic effects with potential adverse psychological consequences is a delicate consideration. Abruptly discontinuing medications that inhibit dopamine and norepinephrine reuptake can lead to withdrawal symptoms. These symptoms may include mood swings, fatigue, and cognitive disturbances. Additionally, some individuals may develop a dependence on these medications, requiring careful management to taper off gradually. Dopamine and norepinephrine play roles in regulating sleep and appetite. Disrupting these neurotransmitters can lead to sleep disturbances, insomnia, or changes in appetite. Monitoring and addressing these side effects are crucial for maintaining overall well-being during the course of treatment. The response to medications that inhibit dopamine and norepinephrine reuptake can vary widely among individuals. Factors such as genetic predispositions, existing medical conditions, and concurrent medications may influence how the body reacts to these interventions. Individualized treatment plans and close monitoring are essential components of responsible prescribing. Impact on Pregnant or Lactating WomenPregnancy and lactation introduce unique considerations when it comes to medication use. The use of Wellbutrin (bupropion) or any other medication during pregnancy requires careful consideration of potential risks and benefits. While studies on the safety of Wellbutrin during pregnancy are inconclusive, there is evidence suggesting a potential association with adverse outcomes, including preterm birth and low birth weight. Additionally, Wellbutrin and its metabolites are present in human breast milk excretions, raising concerns about its impact on nursing infants including alterations in neurotransmitters. Expectant or breastfeeding mothers should engage in thorough discussions with their healthcare providers to weigh the potential benefits against the risks and explore alternative treatment options. Here are some considerations regarding the potential harms of taking Wellbutrin during pregnancy:
Wellbutrin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It's essential for individuals taking Wellbutrin and considering pregnancy, or those who become pregnant while on the medication, to discuss their situation with healthcare professionals. Abruptly stopping antidepressant medication can lead to a recurrence of depressive symptoms, which may have its own set of risks during pregnancy. Healthcare providers will carefully weigh the potential risks and benefits based on the individual's mental health needs. In some cases, healthcare professionals may recommend continuing the medication, adjusting the dosage, or exploring alternative treatments. Pregnant individuals should inform their healthcare providers about their medication use and work collaboratively to make informed decisions that prioritize both maternal mental health and the well-being of the developing fetus. Inactive IngredientsWellbutrin is supplied for oral administration as 75‑mg (yellow‑gold) and 100‑mg (red) film‑coated tablets. Each tablet contains the labeled amount of bupropion hydrochloride and the inactive ingredients:
While the active ingredient in Wellbutrin plays a significant role in its pharmacological effects, it's important not to overlook the inactive ingredients in the formulation. While they make up a smaller portion of the overall product, their cumulative impact should not be underestimated, especially considering the frequent administration of the medication. Though often considered inert, inactive ingredients can still interact with the body in various ways, potentially influencing drug absorption, metabolism, and overall therapeutic efficacy. Moreover, individual sensitivities or allergic reactions to certain inactive ingredients can occur, further emphasizing the importance of evaluating the entire list of components. Even seemingly minor alterations in inactive ingredients can have profound implications, particularly when considering long-term usage. Over time, repeated exposure to these substances may contribute to cumulative effects or unexpected reactions. Therefore, comprehensive scrutiny of all ingredients, active and inactive alike, is essential for a thorough assessment of the safety profile of Wellbutrin and other pharmaceutical products. Impact of Food coloringThe vibrant hues that adorn our favorite processed foods often come from artificial food colorings, but behind the visual appeal lies a potential risk, particularly for pregnant or lactating women. Artificial food color usually contains petroleum and is manufactured in a chemical process that includes formaldehyde, aniline, hydroxides, and sulfuric acids. Most impurities in the food color are in the form of salts or acids. Sometimes lead, arsenic, and mercury may be present as impurities. The U.S. FDA is yet to study the effects of synthetic dyes on behavior in children. To date, there are numerous scientific articles highlighting the relationship of consumption of food colorings to the following health conditions:
Underlying these conditions are the documented mechanics of action that cause physiologic imbalance, which include:
Research has raised concerns about the consumption of food colorings and their potential adverse effects, including implications for conditions like ADHD, autism, and gastrointestinal dysfunction. There is a substantial amount of data exploring the connection between artificial food colorings and ADHD. Researchers suggest that certain artificial colorings may exacerbate hyperactivity and inattention in children with ADHD. Pregnant women, mindful of their diet's impact on fetal development, may choose to limit the intake of foods containing these colorings. The relationship between food colorings and autism spectrum disorders (and the underlying inflammation in the brain) is a topic of ongoing investigation. Meta-analysis have documented a link, emphasizing the need for caution, especially during pregnancy and lactation. As the developing brain is susceptible to external influences, limiting exposure to artificial additives becomes a consideration for expectant and nursing mothers. Artificial food colorings have been associated with gastrointestinal disturbances, ranging from discomfort to more severe issues. Pregnant women, already navigating changes in digestion due to hormonal shifts, may choose to minimize exposure to food colorings to promote digestive well-being during this crucial period. Potential Harms for Pregnant and Lactating Women:
For pregnant or lactating women concerned about the potential harms of food colorings, opting for a diet rich in whole, unprocessed foods becomes a valuable strategy. Choosing fruits, vegetables, and other naturally colorful sources can provide vibrant flavors without the need for artificial additives. While the connection between food colorings and adverse health outcomes is an area of ongoing research, pregnant and lactating women may choose to err on the side of caution. Prioritizing a diet that minimizes artificial additives and focuses on nutrient-dense, whole foods is a proactive step toward supporting both maternal and fetal health. Always consult with healthcare professionals for personalized guidance based on individual circumstances and health considerations. Impact of PEGPolyethylene glycol (PEG) is considered "generally recognized as safe" (GRAS) when used in specific contexts, but some individuals may experience adverse effects. The concerns that arise with the safety of consuming PEG are multifold, including:
Depending on manufacturing processes, PEGs may be contaminated with measurable amounts of ethylene oxide and 1,4-dioxane. The International Agency for Research on Cancer classifies ethylene oxide as a known human carcinogen and 1,4-dioxane as a possible human carcinogen. Ethylene oxide can also harm the nervous system and the California Environmental Protection Agency has classified it as a developmental toxicant based on evidence that it may interfere with human development. 1,4-dioxane is also persistent. In other words, it doesn’t easily degrade and can remain in the environment long after it is rinsed down the shower drain. 1,4-dioxane can be removed from cosmetics during the manufacturing process by vacuum stripping, but there is no easy way for consumers to know whether products containing PEGs have undergone this process.In a study of personal care products marketed as “natural” or “organic” (uncertified), U.S. researchers found 1,4-dioxane as a contaminant in 46 of 100 products analyzed. While carcinogenic contaminants are the primary concern, PEG compounds themselves show some evidence of genotoxicity and if used on broken skin can cause irritation and systemic toxicity. PEG itself is classified as expected to be toxic or harmful as mentioned on the Environment Canada Domestic Substance List. The industry panel that reviews the safety of cosmetics ingredients concluded that some PEG compounds are not safe for use on damaged skin (although the assessment generally approved of the use of these chemicals in cosmetics). Also, PEG functions as a “penetration enhancer,” increasing the permeability of the skin to allow greater absorption of the ingredients — including harmful ingredients. Researchers have observed that a large percentage of parents, caregivers, and practitioners described an explosion of neurological side effects seemingly correlated to polyethylene glycol administration. Those side effects include:
Commonly found in various products, such as medications, laxatives, and skincare items, PEG may lead to the following potential harms:
People with pre-existing health conditions, especially those affecting the kidneys, should inform their healthcare providers before using PEG-containing products. As with any substance, individual responses to PEG can vary, and people experiencing adverse effects should seek medical attention promptly. It's important to weigh the risks and benefits of using PEG-based products based on individual health conditions and circumstances. Impact of TalcTalc is a mineral commonly used in various products such as talcum powder, cosmetics, and personal care items. While talc is considered GRAS for external use, there have been concerns and controversies regarding potential health risks associated with its use, primarily when used in certain ways or in specific product formulations. Here are some of the concerns related to talc:
Individuals concerned about the potential risks of talc-containing products should consider alternatives. As with any substance, moderation and careful use are advisable, and individuals experiencing adverse effects should seek medical advice. Impact of titanium dioxideTitanium dioxide is a widely used pigment and additive in various products, including cosmetics, sunscreens, paints, and food items. While it is generally recognized as safe when used in approved applications, there are concerns about potential health risks associated with certain forms and uses of titanium dioxide. Here are some considerations:
It's important to note that regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA), have assessed the safety of titanium dioxide in approved uses. The permissible limits and specifications vary depending on the application. Consumers concerned about titanium dioxide can choose products with alternatives or consult with healthcare professionals. As research continues, regulatory agencies may update guidelines to ensure the safe use of titanium dioxide in various products. Root Causes vs. MedicationIt's crucial to recognize that the conditions for which Wellbutrin is prescribed—ADHD, anxiety, obesity, and bipolar disorder—may not be caused by a deficiency of Wellbutrin itself. Rather, these conditions are complex and multifaceted, often influenced by underlying nutrition and lifestyle factors. Addressing these root causes is fundamental to comprehensive and sustainable mental health care. Nutrition and Lifestyle Factors: Unveiling the Roots of DepressionDepression, a complex and pervasive mental health condition, often finds its roots in a myriad of factors, extending beyond the realm of neurochemistry to include nutrition and lifestyle elements. Understanding and addressing these underlying factors can play a pivotal role in comprehensive depression management. Here's a closer look at the nutrition and lifestyle aspects that contribute to this intricate mental health landscape: 1. Dietary Choices:
Nutrition and Lifestyle Factors: Off-Label Uses
DeprescriptionAs with many psychotropic medications, Wellbutrin (bupropion) requires careful consideration when discontinuing to minimize the risk of withdrawal symptoms. Abruptly stopping Wellbutrin, also known as going "cold turkey," can lead to uncomfortable and potentially distressing withdrawal symptoms. It is crucial to approach the discontinuation of Wellbutrin with a gradual tapering process, personalized to individual needs, to ensure a smoother transition. Withdrawal symptoms, often associated with sudden cessation of psychotropic drugs, can manifest as a range of physical and psychological discomforts. These may include dizziness, headaches, irritability, mood swings, insomnia, and flu-like symptoms. The severity and duration of withdrawal symptoms can vary from person to person. To mitigate the risk of withdrawal symptoms, the American Psychiatric Association recommends a tapering approach for all antidepressants, including Wellbutrin. Tapering involves gradually reducing the dosage over a specified period, allowing the body to adjust to the decreasing levels of the medication. It is paramount not to discontinue Wellbutrin without consulting your healthcare provider. Your doctor can create a personalized taper schedule based on factors such as the duration of your medication use, your current dosage, and any specific symptoms you may be experiencing. Tapering is typically done over 6 to 8 weeks to provide a gradual adjustment. Every individual responds differently to medication changes. Your doctor can tailor the taper schedule to your unique needs, ensuring a careful balance between minimizing withdrawal symptoms and maintaining mental health stability. If you begin to experience withdrawal symptoms during the tapering process, it is crucial to communicate promptly with your healthcare provider. They may need to reassess your taper schedule or make adjustments based on your symptoms. Restarting Wellbutrin can often alleviate withdrawal symptoms within a few days. The journey of tapering off Wellbutrin is a collaborative effort between you and your healthcare provider. Open communication about your experiences and any emerging symptoms allows for adjustments that prioritize your well-being throughout the process. In the realm of psychotropic medications, a thoughtful and gradual approach to discontinuation is key. Tapering off Wellbutrin under the guidance of your healthcare provider not only minimizes the risk of withdrawal symptoms but also ensures a smoother transition, prioritizing your mental health. Remember, your doctor is your ally in this process, and together, you can navigate the complexities of tapering to promote your overall well-being. ConclusionWhile Wellbutrin has proven efficacy in certain contexts, it's imperative to approach its use with caution, especially considering its potential impacts on neurotransmitter modulation. Pregnant or lactating women should consult their healthcare providers to make informed decisions based on their unique circumstances. Moreover, recognizing that the indications for Wellbutrin may be rooted in broader lifestyle and nutritional factors encourages a more comprehensive approach to mental health care. Collaborative discussions between patients and healthcare professionals can pave the way for personalized and holistic treatment plans that address the underlying causes of mental health conditions. references“Bupropion - Drug Usage Statistics, ClinCalc DrugStats Database.” Clincalc.com, 2021, clincalc.com/DrugStats/Drugs/Bupropion. Medical Expenditure Panel Survey (MEPS) 2013-2021. Agency for Healthcare Research and Quality (AHRQ), Rockville, MD. ClinCalc DrugStats Database version 2024.01.
“Wellbutrin: Package Insert / Prescribing Information.” Drugs.com, www.drugs.com/pro/wellbutrin.html. Starr P, Klein-Schwartz W, Spiller H, Kern P, Ekleberry SE, Kunkel S. Incidence and onset of delayed seizures after overdoses of extended-release bupropion. Am J Emerg Med. 2009 Oct;27(8):911-5. doi: 10.1016/j.ajem.2008.07.004. PMID: 19857406. Spiller, Henry A., et al. “Bupropion Overdose: A 3-Year Multi-Center Retrospective Analysis.” The American Journal of Emergency Medicine, vol. 12, no. 1, Jan. 1994, pp. 43–45, https://doi.org/10.1016/0735-6757(94)90195-3. Accessed 28 Nov. 2021. Bakthavachalu, Prabasheela, et al. “Food Color and Autism: A Meta-Analysis.” Advances in Neurobiology, vol. 24, 2020, pp. 481–504, pubmed.ncbi.nlm.nih.gov/32006369/#:~:text=Many%20families%20with%20autistic%20children, https://doi.org/10.1007/978-3-030-30402-7_15. Hussain, Sunny Z., et al. “Probable Neuropsychiatric Toxicity of Polyethylene Glycol: Roles of Media, Internet and the Caregivers.” GastroHep, vol. 1, no. 3, May 2019, pp. 118–123, https://doi.org/10.1002/ygh2.336. Sellaturay, Priya, et al. “Polyethylene Glycol–Induced Systemic Allergic Reactions (Anaphylaxis).” The Journal of Allergy and Clinical Immunology: In Practice, Oct. 2020, https://doi.org/10.1016/j.jaip.2020.09.029. “Drugs & Medications.” Webmd.com, 2019, www.webmd.com/drugs/2/drug-17118/polyethylene-glycol-3350-oral/details. Black RE, Hurley FJ, and Havery DC. “Occurrence of 1,4-dioxane in cosmetic raw materials and finished cosmetic products.” Int J PharJ AOAC Int. 84, 3 (May-Jun 2001):666-70. Brashear, A. et al. “Ethylene oxide neurotoxicity: a cluster of 12 nurses with peripheral and central nervous system toxicity.” Neurology 46, 4 (Apr 1996):992-8. California. EPA. Office of Environmental Health Hazard Assessment. Chemicals Known to the State to Cause Cancer or Reproductive Toxicity. February 5, 2010.https://www.oehha.org/prop65/prop65_list/files/P65single020510.pdf Environmental Health Association of Nova Scotia. Guide to Less Toxic Products.Halifax: EHANS, 2004. https://www.lesstoxicguide.ca/index.asp?fetch=personal#commo. OCA (Organic Consumer Association). 2008. Consumer alert. Cancer-causing 1,4-dioxane found in personal care products misleadingly branded as natural and organic. Available: https://www.organicconsumers.org/bodycare/DioxaneRelease08.cfm Wangenheim J and Bolcsfoldi G. “Mouse lymphoma L5178Y thymidine kinase locus assay of 50 compounds.” Mutagenesis 3, 3 (May 1988):193-205. Biondi O, Motta S, and Mosesso P. “Low molecular weight polyethylene glycol induces chromosome aberrations in Chinese hamster cells cultured in vitro.” _Mutagenesis_17, 3 (May 2002):261-4. Lanigan, RS (CIR Expert Panel). “Final report on the safety assessment of PPG-11 and PPG-15 stearyl ethers.” Int J Toxicol.20 Suppl 4 (2001):13-26 Cosmetic Ingredient Review. Ingredient Reports — Quick Reference Table (summarizing publications through Dec 2009). https://www.cir-safety.org/staff_files/PublicationsListDec2009.pdf Epstein, S with Fitzgerald, R. Toxic Beauty. Dallas: BenBella Books, 2009: 158-9. Chen, Tao , et al. “Genotoxicity of Titanium Dioxide Nanoparticles.” Journal of Food and Drug Analysis, vol. 22, no. 1, 1 Mar. 2014, pp. 95–104, https://www.sciencedirect.com/science/article/pii/S102194981400009X, https://doi.org/10.1016/j.jfda.2014.01.008.
0 Comments
Leave a Reply. |
The Awareness domain contains research, news, information, observations, and ideas at the level of self in an effort to intellectualize health concepts.
The Lifestyle domain builds off intellectual concepts and offers practical applications.
Taking care of yourself is at the core of the other domains because the others depend on your health and wellness.
Archives
May 2024
Categories
All
|
 RSS Feed
RSS Feed

