|
Researchers have observed in studies analyzing performance that sensory functions are not impaired, but rather functions of perception are significantly altered by cannabis, ultimately diminishing concentration and attention span of the user (Hilderbrand, 2011). The molecules that interact with the body to cause these effects occur via (cannabinoid) receptors found with the membranes of cells throughout the body: in the brain, organs, connective tissues, glands and immune cells (Sulak, 2017). The endocannabinoid system is a endogenous network of receptors maintains a stable internal environment. Certain receptors (CB1 and CB2) within this network have been observed to carry important roles in many processes, including metabolic regulation, craving, pain, anxiety, bone growth, and immune function (Mackie, 2006).
|
Administration of Cannabis
The method of administering cannabis varies among users. Smoking cannabis causes many users to experience symptoms of bronchitis, including wheezing, coughing and chest tightness. Although, research suggest that vaporizers adequately reduce the risk of these pulmonary symptoms. Vaporizers are electronic devices that generate vapor; in this case, by drawing heated air over the plant without igniting it, thus releasing active compounds (cannabinoids) in a vapor form that is relatively free from byproducts of combustion. Vaporizers actually produce more active cannabis compounds compared to the smoke of cannabis that was burned (Loflin & Earleywine, 2015). One group of researchers observed, in a supervised protocol, a 2-week aerobic training program can reduce use of cannabis, in those are considered dependent on cannabis (Buchowski, 2011). In light of the growing population using cannabis, it should be noted that cannabis use is a much safer alternative than pharmaceutical drugs.
May Manage Type-2 Diabetes & Obesity
Evidence suggests that the endocannabinoid system regulates food intake and energy homeostasis, and that overactivation of this system is associated with T2DM and obesity. Researchers have observed that regulating cannabinoid receptor (CB1) caused a significant decrease in body weight, waist circumference, triglyceride concentrations, and HbA1c, and an increase in HDLs and adiponectin concentrations. Cannabidiol (CBD), one of the major cannabinoids present in Cannabis sativa L., has multiple therapeutic effects on hyperglycemia, via various receptors, such as the orphan G-protein-coupled receptor-55 (GPR55), the endothelial cannabinoid receptor, the transient receptor potential vanilloid 1 (TRPV1) receptor, a1-adrenoceptors, m opioid receptors, the adenosine transporter, and serotonin-1A receptors, exhibiting anti-inflammatory and anti-oxidative properties. Through experiments conducted on obese rodents, researchers observed that administration of CBD (3 mg/kg/body weight) for 4 weeks resulted in significantly increased levels of HDLs (good cholesterol) by 55% and both liver glycogen and adiponectin concentrations, as well as reduced total cholesterol by >25% and liver triglycerides.
Intraperitoneal administration of Δ9-Tetrahydrocannabivarin (THCV) (3, 10, 30 mg/kg/body weight), a naturally occurring derivative of tetrahydrocannabinol, acting on both CB1 and CB2 receptors, also been observed to result in decreased feeding and weight loss in rodents. A similar study observed that oral administration of THCV (2.5-15.5 mg/kg/body weight) to diet-induced obese mice, significantly reduced body fat content and liver triglycerides, increased energy expenditure, and reduced fasting insulin levels, as well as reduced the 30-minute insulin response to oral glucose. The researchers observed that a 1:1 ratio of THCV and CBD reduced total cholesterol levels by 19%, liver triglycerides, fasting insulin, and increased HDLs by 50%, liver glycogen levels and increased energy expenditure. Extrapolating this information into an experiment on patients with T2DM, researchers observed that administration of THCV (5 mg twice daily) significantly decreased fasting plasma glucose concentrations and improved b-cell function, compared to a placebo. These results suggest that THCV, present in Cannabis sativa L., may offer safe and effective therapeutic effects in individuals with T2DM (Jadoon et al., 2016).
Intraperitoneal administration of Δ9-Tetrahydrocannabivarin (THCV) (3, 10, 30 mg/kg/body weight), a naturally occurring derivative of tetrahydrocannabinol, acting on both CB1 and CB2 receptors, also been observed to result in decreased feeding and weight loss in rodents. A similar study observed that oral administration of THCV (2.5-15.5 mg/kg/body weight) to diet-induced obese mice, significantly reduced body fat content and liver triglycerides, increased energy expenditure, and reduced fasting insulin levels, as well as reduced the 30-minute insulin response to oral glucose. The researchers observed that a 1:1 ratio of THCV and CBD reduced total cholesterol levels by 19%, liver triglycerides, fasting insulin, and increased HDLs by 50%, liver glycogen levels and increased energy expenditure. Extrapolating this information into an experiment on patients with T2DM, researchers observed that administration of THCV (5 mg twice daily) significantly decreased fasting plasma glucose concentrations and improved b-cell function, compared to a placebo. These results suggest that THCV, present in Cannabis sativa L., may offer safe and effective therapeutic effects in individuals with T2DM (Jadoon et al., 2016).
Influence on Age-Related Cognitive Function
The process and progression of aging is determined by the balance of detrimental, randomly determined processes. Substantial evidence suggests that the endocannabinoid system regulates the physiological processes underlying aging. As the activity of endocannabinoid system decreases during aging, the ability of the brain to express CB1 receptors reduces. Researchers have examined the effects of prolonged exposure to low concentrations of Δ9-tetrahydrocannabinol (THC) on learning and memory performance in mice of various ages (2, 12, 18 months). THC was administered to the mice via implanted osmotic miniature pumps able to release 3 mg per kilogram of body weight per day for 28 days. The researchers observed that low concentrations of THC reversed the age-related decline in cognitive performance of mice (12 and 18 months). This result was accompanied by the enhanced expression of synaptic marker proteins and increased hippocampal spine density. Furthermore, administration of THC to mice (aged 12 months) restored hippocampal gene transcription patterns that closely resembled those of the THC-free animals (aged 2 months). Therefore, restoring CB1 signaling in old individuals may be an effective strategy to manage age-related cognitive deficits (Bilkei-Gorzo et al., 2017).
Influence on Dementia
Alzheimer's disease is the leading cause of dementia among the elderly, and with the ever-increasing size of this population, cases of Alzheimer's disease are expected to triple over the next 50 years. Consequently, the development of treatments that slow or halt the disease progression have become imperative to both improve the quality of life for patients as well as reduce the health care costs attributable to Alzheimer's disease. Researchers have observed that the active component of marijuana, THC, competitively inhibits the enzyme acetylcholinesterase (AChE) as well as prevents AChE-induced amyloid β-peptide (Aβ) aggregation, the key pathological marker of Alzheimer's disease. Compared to currently approved drugs prescribed for the treatment of Alzheimer's disease, THC is a considerably superior inhibitor of Aβ aggregation, and this evidence provides a previously unrecognized molecular mechanism through which cannabinoid molecules may directly impact the progression of this debilitating disease (Eubanks et al., 2006).
Safety of Cannabis
|
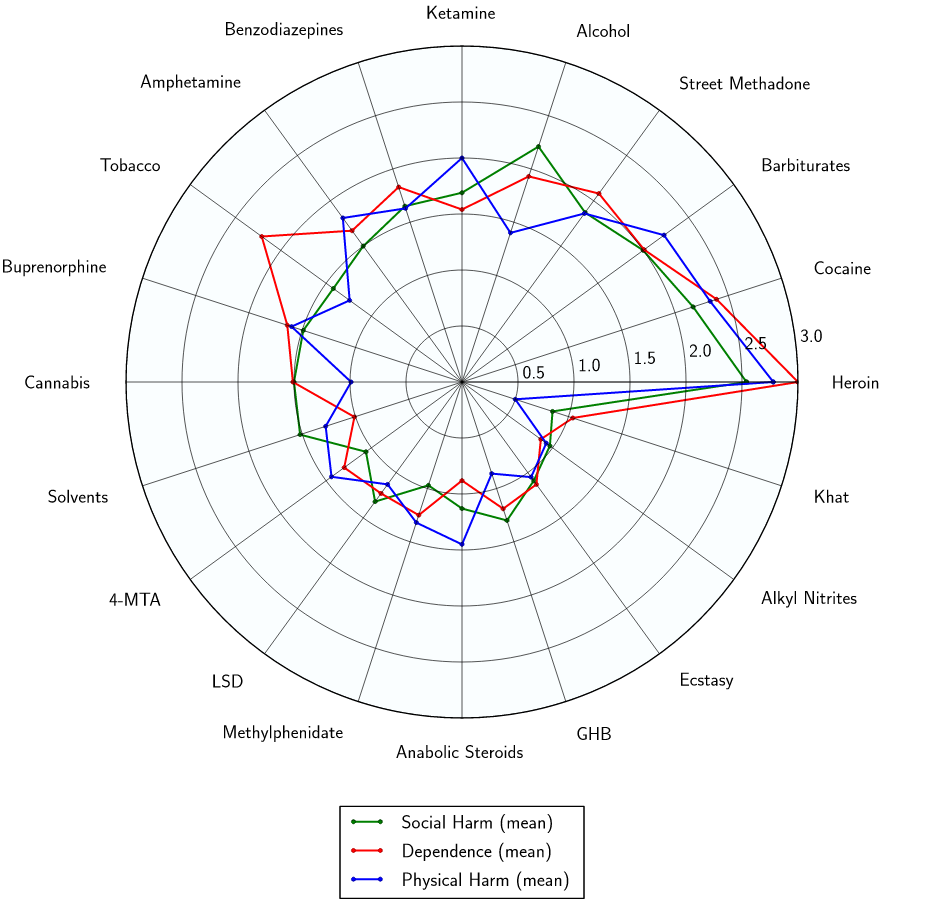
Harmful drugs are regulated according to classification systems that purport to relate to the harms and risks of each drug. However, the methodology and processes underlying classification systems are generally neither specified nor transparent, which reduces confidence in their accuracy and undermines health education messages. Researchers have developed and explored the feasibility of the use of a nine-category matrix of harm to assess the harms of a range of illicit drugs in an evidence-based fashion. The assessment of harm parameters include physical harm (acute, chronic, and intravenous), dependence (intensity of pleasure, psychological and physical dependence), and social harms (intoxication, health-care costs, and other social harms). Interestingly, cannabis scored safer than alcohol and tobacco, which are both in the top ten, higher-harm group (Nutt, King, Saulsbury & Blakemore, 2007).
|
“Cannabis is anathema to the dominator culture because it deconditions or decouples users from accepted values. Because of its subliminally psychedelic effect, cannabis, when pursued as a lifestyle, places a person in intuitive contact with less goal-oriented and less competitive behavior patterns. For these reasons marijuana is unwelcome in the modern office environment, while a drug such as coffee, which reinforces the values of industrial culture, is both welcomed and encouraged. Cannabis use is correctly sensed as heretical and deeply disloyal to the values of male dominance and stratified hierarchy. Legalization of marijuana is thus a complex issue, since it involves legitimating a social factor that might ameliorate or even modify ego-dominant values.”
Terence McKenna
References
Bilkei-Gorzo, A., Albayram, O., Draffehn, A., Michel, K., Piyanova, A., & Oppenheimer, H. et al. (2017). A chronic low dose of Δ9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nature Medicine, 23(6), 782-787. http://dx.doi.org/10.1038/nm.4311
Buchowski, M. , Martin, M. , Meade, N. , Charboneau, E. , Park, S. , et al. (2011). Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PLoS ONE, 6(3), e17465
Eubanks, L., Rogers, C., Beuscher, Koob, G., Olson, A., Dickerson, T. and Janda, K. (2006). A Molecular Link between the Active Component of Marijuana and Alzheimer's Disease Pathology. Molecular Pharmaceutics, 3(6), pp.773-777. https://doi.org/10.1021/mp060066m
Hilderbrand, R. (2011). High-Performance Sport, Marijuana, and Cannabimimetics. Journal Of Analytical Toxicology, 35(9), 624-637. http://dx.doi.org/10.1093/anatox/35.9.624
Jadoon, K., Ratcliffe, S., Barrett, D., Thomas, E., Stott, C., & Bell, J. et al. (2016). Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care, 39(10), 1777-1786. http://dx.doi.org/10.2337/dc16-0650
Loflin, M., & Earleywine, M. (2015). No smoke, no fire: What the initial literature suggests regarding vapourized cannabis and respiratory risk. Canadian Journal of Respiratory Therapy: CJRT = Revue Canadienne de La Thérapie Respiratoire : RCTR, 51(1), 7–9.
Nutt, D., King, L., Saulsbury, W., & Blakemore, C. (2007). Development of a rational scale to assess the harm of drugs of potential misuse. The Lancet, 369(9566), 1047-1053. http://dx.doi.org/10.1016/s0140-6736(07)60464-4
Sulak, D. (2017). Introduction to the Endocannabinoid System - NORML.org - Working to Reform Marijuana Laws. Norml.org. Retrieved 7 April 2017, from http://norml.org/library/item/introduction-to-the-endocannabinoid-system
Mackie, K. (2006). CANNABINOID RECEPTORS AS THERAPEUTIC TARGETS. Annual Review Of Pharmacology And Toxicology, 46(1), 101-122. http://dx.doi.org/10.1146/annurev.pharmtox.46.120604.141254
Buchowski, M. , Martin, M. , Meade, N. , Charboneau, E. , Park, S. , et al. (2011). Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PLoS ONE, 6(3), e17465
Eubanks, L., Rogers, C., Beuscher, Koob, G., Olson, A., Dickerson, T. and Janda, K. (2006). A Molecular Link between the Active Component of Marijuana and Alzheimer's Disease Pathology. Molecular Pharmaceutics, 3(6), pp.773-777. https://doi.org/10.1021/mp060066m
Hilderbrand, R. (2011). High-Performance Sport, Marijuana, and Cannabimimetics. Journal Of Analytical Toxicology, 35(9), 624-637. http://dx.doi.org/10.1093/anatox/35.9.624
Jadoon, K., Ratcliffe, S., Barrett, D., Thomas, E., Stott, C., & Bell, J. et al. (2016). Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care, 39(10), 1777-1786. http://dx.doi.org/10.2337/dc16-0650
Loflin, M., & Earleywine, M. (2015). No smoke, no fire: What the initial literature suggests regarding vapourized cannabis and respiratory risk. Canadian Journal of Respiratory Therapy: CJRT = Revue Canadienne de La Thérapie Respiratoire : RCTR, 51(1), 7–9.
Nutt, D., King, L., Saulsbury, W., & Blakemore, C. (2007). Development of a rational scale to assess the harm of drugs of potential misuse. The Lancet, 369(9566), 1047-1053. http://dx.doi.org/10.1016/s0140-6736(07)60464-4
Sulak, D. (2017). Introduction to the Endocannabinoid System - NORML.org - Working to Reform Marijuana Laws. Norml.org. Retrieved 7 April 2017, from http://norml.org/library/item/introduction-to-the-endocannabinoid-system
Mackie, K. (2006). CANNABINOID RECEPTORS AS THERAPEUTIC TARGETS. Annual Review Of Pharmacology And Toxicology, 46(1), 101-122. http://dx.doi.org/10.1146/annurev.pharmtox.46.120604.141254