Acid-Base Balance
What is an Acid?
chemical substance that neutralizes alkalis; proton donor
Acidic anions in food include chloride, phosphorous, sulfates, and other organic acids.
Acidic anions in food include chloride, phosphorous, sulfates, and other organic acids.
What is a BAse?
chemical substance capable with reacting with acid to form a salt and water; proton acceptor
Basic/Alkaline cations in food include sodium, potassium, calcium, and magnesium.
Basic/Alkaline cations in food include sodium, potassium, calcium, and magnesium.
What is Potential renal acid load (PRAL)
Potential renal acid load (PRAL) is a calculated value to establish a method of estimating acid loads of foods or diets. "PRAL provides an estimate of the production of endogenous acid that exceeds the level of alkali produced for given amounts of foods ingested daily. The concept of PRAL calculation is physiologically based and takes into account different intestinal absorption rates of individual minerals and of sulfur-containing protein, as well as the amount of sulfate produced from metabolized proteins. This method of calculation was experimentally validated in healthy adults, and it showed that, under controlled conditions, acid loads and renal net acid excretion (NAE) can be reliably estimated from diet composition" (Remer, Dimitriou, & Manz, 2003).
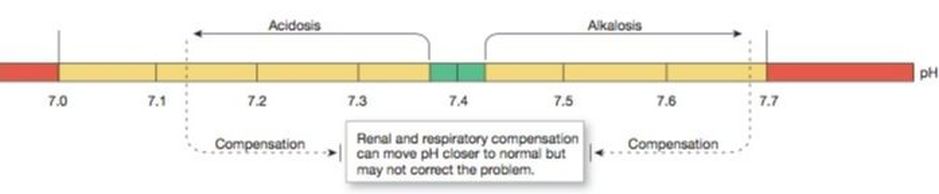
Acid-base balance (pH homeostasis) is an essential, tightly-regulated, bodily function. The pH of a solution is a measure of its H+ (hydrogen ion) concentration. Because the body’s H+ concentration is so low, it is commonly expressed on a logarithmic pH scale of 0–14, in which a pH of 7.0 is neutral (neither acidic nor basic). If the pH of a solution is below 7.0, the solution is considered acidic. If the pH is above 7.0, the solution is considered alkaline (basic). The normal pH of the body is slightly alkaline, and ranges between 7.38 - 7.42, . A change of 1 pH unit represents a 10-fold change in H+ concentration.
Extracellular pH usually reflects intracellular pH, and vice versa. Because monitoring intracellular conditions is difficult, plasma values are used clinically as an indicator of extracellular fluid and whole body pH. Intracellular proteins, such as enzymes and membrane channels, are particularly sensitive to pH because the function of these proteins depends on their three-dimensional shape. Changes in H+ concentration alter the tertiary structure of proteins by interacting with hydrogen bonds in the molecules, disrupting the proteins’ three-dimensional structures and activities.
As we age, the ability of the body to regulate acids and bases gradually declines. There are three mechanisms that regulate plasma pH: buffers, ventilation and renal excretion. If the body fails to keep pH between 7.00 and 7.70, acidosis or alkalosis may be fatal (Johnson, Ober, Garrison, & Silverthorn, 2012).
Extracellular pH usually reflects intracellular pH, and vice versa. Because monitoring intracellular conditions is difficult, plasma values are used clinically as an indicator of extracellular fluid and whole body pH. Intracellular proteins, such as enzymes and membrane channels, are particularly sensitive to pH because the function of these proteins depends on their three-dimensional shape. Changes in H+ concentration alter the tertiary structure of proteins by interacting with hydrogen bonds in the molecules, disrupting the proteins’ three-dimensional structures and activities.
As we age, the ability of the body to regulate acids and bases gradually declines. There are three mechanisms that regulate plasma pH: buffers, ventilation and renal excretion. If the body fails to keep pH between 7.00 and 7.70, acidosis or alkalosis may be fatal (Johnson, Ober, Garrison, & Silverthorn, 2012).
Acid-Base Disturbances
|
Abnormal pH may significantly affect the activity of the nervous system. If pH is too low—the condition known as acidosis—neurons become less excitable, and CNS depression results. Patients become confused and disoriented, then slip into a coma. If CNS depression progresses, the respiratory centers cease to function, causing death.
|
If pH is too high—the condition known as alkalosis— neurons become hyperexcitable, firing action potentials at the slightest signal. This condition shows up first as sensory changes, such as numbness or tingling, then as muscle twitches. If alkalosis is severe, muscle twitches turn into sustained contractions (tetanus) that paralyze respiratory muscles.
|
Acidic and Alkaline Foods
The pH level of a food before digestion, has little to with the acidic or alkaline effects it has on the body. Instead, the resulting pH depends on whether acidic or alkaline byproducts (metabolites) are created once the food is digested and absorbed by the body. For instance, lemons are considered acidic, however, once digested and metabolized, lemons produce alkaline byproducts, which make the blood and urine more alkaline. This is why lemons are thought of as an alkaline food, despite its acidic pH before consumption.
7 pH
|
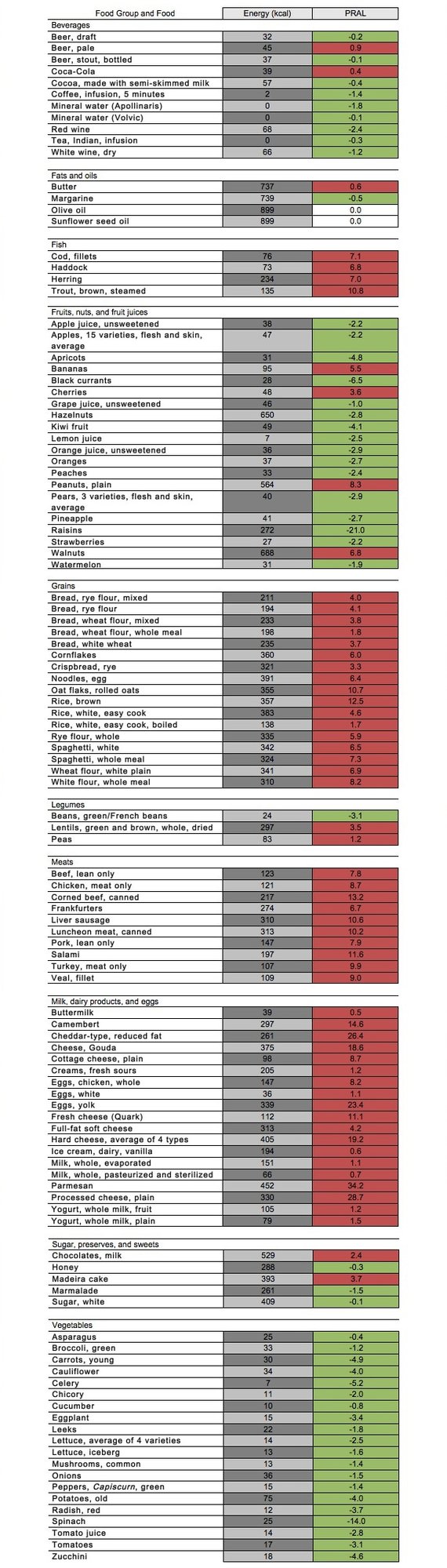
Foods can be categorized either acidifying or alkalizing by their potential renal acid loads (PRALs). A negative PRAL score indicates the food is basic/alkaline. A positive PRAL score indicates the food is acidic. A score of 0 indicates the food is neutral. In general vegetables, fruit, fruit juices, and wine have a negative acid load (alkaline effect). In general, grain products, animal products (e.g., dairy products, eggs, meats, and fish) beer and soda have high acid loads (acidifying effect) (Schwalfenberg, 2011). There are, however, some results that vary. For instance, coffee has a PRAL value of -1.4, meaning a alkaline residue is present, whereas it is generally recognized as an acid-forming food. Below is a list of many different foods and food groups, with the corresponding PRAL value.
Remember...
A negative PRAL score indicates the food is basic/alkaline.
A score of 0 indicates the food is neutral.
A positive PRAL score indicates the food is acidic.
A score of 0 indicates the food is neutral.
A positive PRAL score indicates the food is acidic.
Alkaline Foods Formed the Nutrient Requirements For Our Preagricultural Ancestors
The nutrient requirements of Homo sapiens were established by natural selection over the course of millions of years in which our ancestors consumed foods exclusively from wild animals and uncultivated plants. With the rise of new inventions in agriculture with industrial-scale food production and distribution, these technologies allowed natural selection to become an enormous challenge.
Compared to the diet habitually ingested by preagricultural Homo sapiens, the diet of modern Homo sapiens is rich in saturated fat, simple sugars, sodium, and chloride and poor in fiber, magnesium, and potassium. These along with numerous other postagricultural dietary changes have been implicated as risk factors in the pathogenesis of atherosclerosis, hypertension, type 2 diabetes, osteoporosis, and certain types of cancer.
Researchers compared the pre-agricultural diet of our ancestors to the modern North American diet. After evaluating the two diets using NEAP (net endogenous acid production), a -88mEq/day acid load characterized the pre-agricultural diet while the modern diet was characterized by a +48mEq/day acid load. This means is that our ancestors evolved eating a diet that was very alkaline/basic and low in foods that produce acid. Thus, the modern Western diet which is high in acidic-forming foods, is very different from what we evolved to eat. As a result, the modern diet is responsible for life-long, low grade systemic acidosis (Sebastian, Frassetto, Sellmeyer, Merriam, & Morris, 2002).
Compared to the diet habitually ingested by preagricultural Homo sapiens, the diet of modern Homo sapiens is rich in saturated fat, simple sugars, sodium, and chloride and poor in fiber, magnesium, and potassium. These along with numerous other postagricultural dietary changes have been implicated as risk factors in the pathogenesis of atherosclerosis, hypertension, type 2 diabetes, osteoporosis, and certain types of cancer.
Researchers compared the pre-agricultural diet of our ancestors to the modern North American diet. After evaluating the two diets using NEAP (net endogenous acid production), a -88mEq/day acid load characterized the pre-agricultural diet while the modern diet was characterized by a +48mEq/day acid load. This means is that our ancestors evolved eating a diet that was very alkaline/basic and low in foods that produce acid. Thus, the modern Western diet which is high in acidic-forming foods, is very different from what we evolved to eat. As a result, the modern diet is responsible for life-long, low grade systemic acidosis (Sebastian, Frassetto, Sellmeyer, Merriam, & Morris, 2002).
Influence of pH in Cells, Organs and Membranes
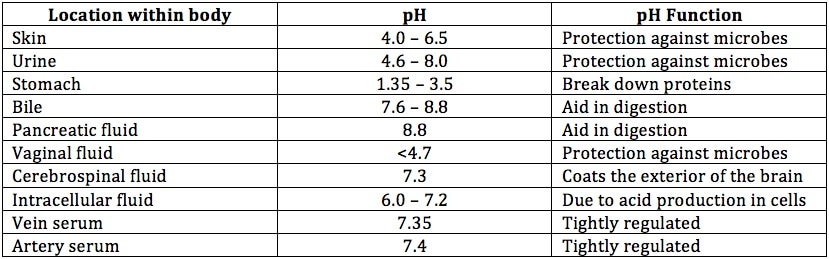
Every cell of the body functions optimally within a certain pH range. The pH throughout the body may vary depending on the location. For instance, the stomach has a pH of 1.35 to 3.5 (the highest acidity) in efforts of digesting food and protection against microbial organisms. However, even within the layers of the stomach exists a basic pH to prevent injury form the acidic conditions. The skin has a pH of 4 to 6.5 to protect against environmental microbes. The pH of the urine may vary from acidic to alkaline depending on the internal environment. Below is a table summarizing the pH levels around some of the locations within the body.
Influence on Bone
Bone is largely composed of calcium, which represents a large reservoir of base within the body. The body responds to acid loads by releasing calcium into circulation to regulate pH. Current estimates suggest the amount of calcium lost in the urine (while consuming the modern Western diet) over time may be as high as almost half the skeletal mass of calcium. Although there are several regulatory factors able to compensate for the loss of calcium in the urine, such as bicarbonate. Excess sodium can also lead to metabolic acidosis, in addition to hypertension and osteoporosis in women. Excess dietary protein may decrease BMD if not buffered by alkaline-rich foods. However, adequate quality protein is advised for the prevention of osteoporosis and sarcopenia (Schwalfenberg, 2011).
Influence on Muscle
Diets that are rich in fruits and vegetables, with a reduced acid load, result in the preservation of muscle mass in older adults. Skeletal muscle breakdown is accelerated by chronic metabolic acidosis. Acute acidosis can be reversed in younger adults by supplementing with sodium bicarbonate (Schwalfenberg, 2011). Although, it should be noted that excessive supplementation of sodium bicarbonate can cause gastrointestinal distress.
Influence on Health
Researchers examined the relationship between dietary acid load, assessed with both the potential renal acid load (PRAL) and the net endogenous acid production (NEAP) scores, and the risk of type 2 diabetes. More than 66,000 women were followed over 14 years for diabetes incidence; 1,372 cases of type-2 diabetes were validated. Of the entire population, individuals with the highest PRAL values, reflecting a greater acid-forming potential, was associated with a significant increase in type 2 diabetes risk, compared to individuals with the lowest PRAL values (Fagherazzi et al., 2013).
Researchers examined over 17,000 Japanese workers between the ages of 19-69 yo. to evaluate the relationship between dietary acid load and markers of insulin resistance (IR). Dietary intake was assessed using a validated brief diet history questionnaire. Potential renal acid load (PRAL) and net endogenous acid production (NEAP) scores were derived from nutrient intake. The results of the study suggest that high dietary acid load is associated with IR among apparently healthy adults (Akter et al., 2016).
Researchers examined the relationship between NEAP scores and the risk of incident hypertension among 87,293 women without a history of hypertension. The researchers found that a higher diet-dependent net acid load was independently associated with the risk of hypertension (Zhang, Curhan, & Forman, 2009).
Researchers examined over 11,000 people from the Korea National Health and Nutrition Examination Survey between 2008–2011 to determine the relationship between cardiovascular disease and dietary acid load. Acid–base balance status was assessed with both the potential renal acid load (PRAL) and the dietary acid load (DAL) scores derived from nutrient intake. The researchers observed that individuals highest PRAL, which corresponds to a diet-induced acid load, was associated with increased risk of cardiovascular disease, independent of obesity, exercise, and insulin resistance (Han et al., 2016).
Researchers examined over 17,000 Japanese workers between the ages of 19-69 yo. to evaluate the relationship between dietary acid load and markers of insulin resistance (IR). Dietary intake was assessed using a validated brief diet history questionnaire. Potential renal acid load (PRAL) and net endogenous acid production (NEAP) scores were derived from nutrient intake. The results of the study suggest that high dietary acid load is associated with IR among apparently healthy adults (Akter et al., 2016).
Researchers examined the relationship between NEAP scores and the risk of incident hypertension among 87,293 women without a history of hypertension. The researchers found that a higher diet-dependent net acid load was independently associated with the risk of hypertension (Zhang, Curhan, & Forman, 2009).
Researchers examined over 11,000 people from the Korea National Health and Nutrition Examination Survey between 2008–2011 to determine the relationship between cardiovascular disease and dietary acid load. Acid–base balance status was assessed with both the potential renal acid load (PRAL) and the dietary acid load (DAL) scores derived from nutrient intake. The researchers observed that individuals highest PRAL, which corresponds to a diet-induced acid load, was associated with increased risk of cardiovascular disease, independent of obesity, exercise, and insulin resistance (Han et al., 2016).
Balance is Key
It has been suggested that an alkaline diet may prevent some diseases and result in drastic health benefits. An alkaline environment may improve health, although moderation is key. A body that is too alkaline, outside of the tightly regulated pH levels, can have its own set of problems.
References
Akter, S., Eguchi, M., Kuwahara, K., Kochi, T., Ito, R., Kurotani, K., … Mizoue, T. (2016). High dietary acid load is associated with insulin resistance: The Furukawa nutrition and health study. Clinical Nutrition, 35(2), 453–459. doi:10.1016/j.clnu.2015.03.008
Fagherazzi, G., Vilier, A., Bonnet, F., Lajous, M., Balkau, B., Boutron-Ruault, M.-C., & Clavel-Chapelon, F. (2013). Dietary acid load and risk of type 2 diabetes: The E3N-EPIC cohort study. Diabetologia, 57(2), 313–320. doi:10.1007/s00125-013-3100-0
Han, E., Kim, G., Hong, N., Lee, Y., Kim, D. W., Shin, H. J., … Cha, B.-S. (2016). Association between dietary acid load and the risk of cardiovascular disease: Nationwide surveys (KNHANES 2008–2011). Cardiovascular Diabetology, 15(1), . doi:10.1186/s12933-016-0436-z
Johnson, B. R., Ober, W. C., Garrison, C. W., & Silverthorn, D. U. (2012). Human physiology: An integrated approach (6th ed.). San Francisco, CA: Benjamin-Cummings Publishing Company, Subs of Addison Wesley Longman.
Remer, T., Dimitriou, T., & Manz, F. (2003). Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. The American Journal of Clinical Nutrition, 77(5), 1255–1260. Retrieved from http://ajcn.nutrition.org/content/77/5/1255.full
Remer, T., & Manz, F. (1995). Potential renal acid load of foods and its influence on urine pH. Journal of the American Dietetic Association., 95(7), 791–7. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7797810
Schwalfenberg, G. K. (2011). The alkaline diet: Is there evidence that an alkaline pH diet benefits health? , 2012, . Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3195546/
Sebastian, A., Frassetto, L. A., Sellmeyer, D. E., Merriam, R. L., & Morris, C. R. (2002). Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. The American Journal of Clinical Nutrition, 76(6), 1308–1316. Retrieved from http://ajcn.nutrition.org/content/76/6/1308.long
Zhang, L., Curhan, G. C., & Forman, J. P. (2009). Diet-dependent net acid load and risk of incident hypertension in United States women. Diet/Obesity, 54(4), 751–755. doi:10.1161/HYPERTENSIONAHA.109.135582
Fagherazzi, G., Vilier, A., Bonnet, F., Lajous, M., Balkau, B., Boutron-Ruault, M.-C., & Clavel-Chapelon, F. (2013). Dietary acid load and risk of type 2 diabetes: The E3N-EPIC cohort study. Diabetologia, 57(2), 313–320. doi:10.1007/s00125-013-3100-0
Han, E., Kim, G., Hong, N., Lee, Y., Kim, D. W., Shin, H. J., … Cha, B.-S. (2016). Association between dietary acid load and the risk of cardiovascular disease: Nationwide surveys (KNHANES 2008–2011). Cardiovascular Diabetology, 15(1), . doi:10.1186/s12933-016-0436-z
Johnson, B. R., Ober, W. C., Garrison, C. W., & Silverthorn, D. U. (2012). Human physiology: An integrated approach (6th ed.). San Francisco, CA: Benjamin-Cummings Publishing Company, Subs of Addison Wesley Longman.
Remer, T., Dimitriou, T., & Manz, F. (2003). Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. The American Journal of Clinical Nutrition, 77(5), 1255–1260. Retrieved from http://ajcn.nutrition.org/content/77/5/1255.full
Remer, T., & Manz, F. (1995). Potential renal acid load of foods and its influence on urine pH. Journal of the American Dietetic Association., 95(7), 791–7. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7797810
Schwalfenberg, G. K. (2011). The alkaline diet: Is there evidence that an alkaline pH diet benefits health? , 2012, . Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3195546/
Sebastian, A., Frassetto, L. A., Sellmeyer, D. E., Merriam, R. L., & Morris, C. R. (2002). Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. The American Journal of Clinical Nutrition, 76(6), 1308–1316. Retrieved from http://ajcn.nutrition.org/content/76/6/1308.long
Zhang, L., Curhan, G. C., & Forman, J. P. (2009). Diet-dependent net acid load and risk of incident hypertension in United States women. Diet/Obesity, 54(4), 751–755. doi:10.1161/HYPERTENSIONAHA.109.135582