the imperative of individualized medicine
In the realm of healthcare, the concept of "one size fits all" has long been applied, particularly in the development and administration of medical products like vaccines. However, the idea that a single approach can universally guarantee safety and effectiveness is increasingly being challenged. Biochemical individuality, the unique biological makeup of each person, sheds light on the limitations of a standardized approach and emphasizes the need for more personalized medical interventions.
Medical products, especially vaccines, are designed with the intention of providing broad protection against specific diseases. This approach has supposedly contributed to significant advancements in public health, and in doing so it assumes a uniform response across diverse populations. Biochemical individuality suggests otherwise.
Every individual possesses a distinct genetic, biochemical, and physiological makeup, influencing how the body processes medications, including vaccines. Factors such as genetic variations, environmental exposures, and lifestyle choices all contribute to this uniqueness. Therefore, a medical intervention that works well for one person may not necessarily yield the same results for another.
Vaccines aim to stimulate the immune system (typically using toxic adjuvants, such as aluminum), creating a protective response against pathogens. However, the efficacy and safety of vaccines can vary among individuals due to their unique biological characteristics. Factors influencing vaccine response include:
Recognizing the limitations of a "one size fits all" approach emphasizes the importance of moving toward personalized medicine. Tailoring medical interventions based on an individual's unique characteristics ensures better safety and efficacy. Precision medicine considers factors such as genetics, lifestyle, and environment to deliver targeted and optimized healthcare.
Biochemical individuality challenges the conventional approach of applying medical interventions universally. In the case of vaccines, acknowledging the diverse responses among individuals underscores the importance of personalized medicine. As we strive for advancements in healthcare, embracing the uniqueness of each person's biology is key to achieving safer and more effective medical outcomes.
Medical products, especially vaccines, are designed with the intention of providing broad protection against specific diseases. This approach has supposedly contributed to significant advancements in public health, and in doing so it assumes a uniform response across diverse populations. Biochemical individuality suggests otherwise.
Every individual possesses a distinct genetic, biochemical, and physiological makeup, influencing how the body processes medications, including vaccines. Factors such as genetic variations, environmental exposures, and lifestyle choices all contribute to this uniqueness. Therefore, a medical intervention that works well for one person may not necessarily yield the same results for another.
Vaccines aim to stimulate the immune system (typically using toxic adjuvants, such as aluminum), creating a protective response against pathogens. However, the efficacy and safety of vaccines can vary among individuals due to their unique biological characteristics. Factors influencing vaccine response include:
- Genetic Variability: Genetic differences can affect how the immune system recognizes and responds to vaccines. Variations in immune-related genes may lead to diverse reactions to vaccination.
- Metabolic Variances: Individual variations in metabolic pathways can influence the processing and elimination of vaccine components. Factors such as liver function and enzyme activity contribute to these differences.
- Immunological Diversity: The immune system's complexity means that individuals may mount varied responses to vaccines. Factors like pre-existing immunity, age, and overall health can impact the strength and duration of the immune response.
- Environmental Factors: Exposures to environmental toxins, diet, and lifestyle choices contribute to biochemical individuality, affecting how the body interacts with vaccines.
Recognizing the limitations of a "one size fits all" approach emphasizes the importance of moving toward personalized medicine. Tailoring medical interventions based on an individual's unique characteristics ensures better safety and efficacy. Precision medicine considers factors such as genetics, lifestyle, and environment to deliver targeted and optimized healthcare.
Biochemical individuality challenges the conventional approach of applying medical interventions universally. In the case of vaccines, acknowledging the diverse responses among individuals underscores the importance of personalized medicine. As we strive for advancements in healthcare, embracing the uniqueness of each person's biology is key to achieving safer and more effective medical outcomes.
In defense of informed consent
In the ongoing discourse surrounding vaccines, it is crucial to emphasize a nuanced perspective that prioritizes health and individual choice. The stance presented here is not anti-vaccine but pro-health, rooted in the belief that informed decisions and personal autonomy should guide medical choices. It questions the lack of rigorous testing, legal exemptions for manufacturers, and the imposition of mandates.
1. Unavoidably Unsafe: Supreme Court Ruling and Vaccine Manufacturers
The 2010 Supreme Court ruling declaring vaccines as "unavoidably unsafe" highlights a critical aspect of these pharmaceutical products. With the claim that vaccines undoubtedly contribute to public health, the acknowledgment of inherent risks prompts a closer examination of their safety profiles.
1. Unavoidably Unsafe: Supreme Court Ruling and Vaccine Manufacturers
The 2010 Supreme Court ruling declaring vaccines as "unavoidably unsafe" highlights a critical aspect of these pharmaceutical products. With the claim that vaccines undoubtedly contribute to public health, the acknowledgment of inherent risks prompts a closer examination of their safety profiles.
| BRUESEWITZ ET AL. v. WYETH LLC, FKA WYETH, INC., ET AL. (Oct 2010).pdf | |
| File Size: | 79 kb |
| File Type: | |
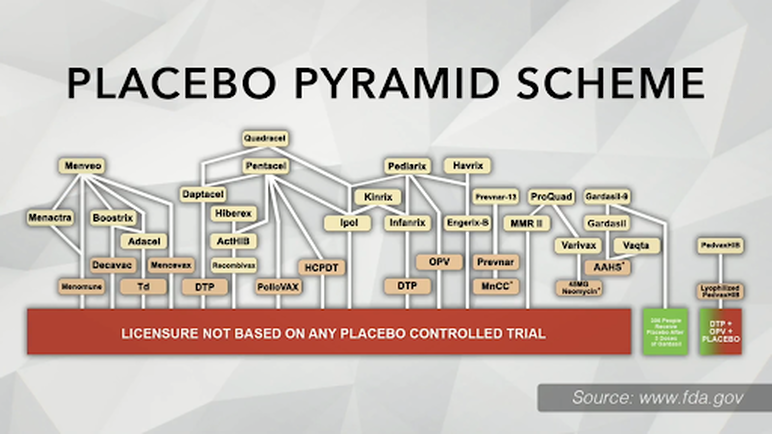
2. Lack of Legitimate Placebo-Controlled Trials
One of the fundamental concerns raised is the absence of legitimate placebo-controlled trials before vaccines (literally, every single one of them) are released into the market. This deviation from standard testing procedures raises questions about the thorough evaluation of potential side effects and long-term impacts. Often times, the safety profile is assess for days or weeks, not months, not years - Considering the rampant increase in chronic disease rates worldwide, is that enough time to determine if any pharmaceutical product causes harm in the long term?
One of the fundamental concerns raised is the absence of legitimate placebo-controlled trials before vaccines (literally, every single one of them) are released into the market. This deviation from standard testing procedures raises questions about the thorough evaluation of potential side effects and long-term impacts. Often times, the safety profile is assess for days or weeks, not months, not years - Considering the rampant increase in chronic disease rates worldwide, is that enough time to determine if any pharmaceutical product causes harm in the long term?
Your browser does not support viewing this document. Click here to download the document.
3. Manufacturer Exemptions and Liability
A notable aspect of the vaccine landscape is the legal immunity granted to manufacturers, which is unlike any other product in the world. The absence of liability in the event of harm from their products raises eyebrows, as it seemingly contradicts the principle of accountability for pharmaceutical interventions. This exemption diminishes the incentive for manufacturers to rigorously ensure the safety of their products.
A notable aspect of the vaccine landscape is the legal immunity granted to manufacturers, which is unlike any other product in the world. The absence of liability in the event of harm from their products raises eyebrows, as it seemingly contradicts the principle of accountability for pharmaceutical interventions. This exemption diminishes the incentive for manufacturers to rigorously ensure the safety of their products.
4. Informed Choice Over Mandates
Given the aforementioned information, if one still "believes" (despite the lack of evidence) that these products are safe and effective, then they should have the right to choose - the emphasis here lies on the right to make informed choices regarding one's health. Advocating for personal autonomy, the stance opposes mandates that compel individuals to receive vaccines without considering their unique health circumstances, values, or concerns.
5. Free Will and Personal Decision-Making
In a free world, the right to make personal health decisions remains paramount. Acknowledging individual agency, this perspective respects the choice of those who opt for vaccination while asserting the need for transparency, accountability, and a robust evaluation of risks.
The stance presented here reflects a commitment to health, individual choice, and informed decision-making. It challenges the status quo by questioning the lack of thorough testing, manufacturer exemptions, and the imposition of mandates. By promoting dialogue and advocating for transparency in the realm of vaccines, it seeks to empower individuals to make choices aligned with their values and well-being.
Given the aforementioned information, if one still "believes" (despite the lack of evidence) that these products are safe and effective, then they should have the right to choose - the emphasis here lies on the right to make informed choices regarding one's health. Advocating for personal autonomy, the stance opposes mandates that compel individuals to receive vaccines without considering their unique health circumstances, values, or concerns.
5. Free Will and Personal Decision-Making
In a free world, the right to make personal health decisions remains paramount. Acknowledging individual agency, this perspective respects the choice of those who opt for vaccination while asserting the need for transparency, accountability, and a robust evaluation of risks.
The stance presented here reflects a commitment to health, individual choice, and informed decision-making. It challenges the status quo by questioning the lack of thorough testing, manufacturer exemptions, and the imposition of mandates. By promoting dialogue and advocating for transparency in the realm of vaccines, it seeks to empower individuals to make choices aligned with their values and well-being.
vaccine experts under oath
Del Bigtree, joins ICAN Lead Counsel, Aaron Siri, Esq., on the stage of Freedom Fest, in Memphis, TN, where they present ‘Vaccine Experts Under Oath: Shocking Revelations in the Fight for Transparency And Truth.’ Everything you thought you knew about vaccines, is wrong. Listen to Del and Aaron take you through court depositions and cross examinations of the world’s leading vaccine experts. Listen to shocking admissions by these experts, in their own words, when they are compelled to tell the truth, under oath.
safegaurding vaccine excemptions
In a recent presentation to the Arizona State Legislature, attorney Aaron Siri voiced compelling arguments against vaccine mandates, emphasizing their oppressive nature and the importance of preserving individual freedom. Siri's insights shed light on the downsides of vaccines, drawing from historical context and scientific studies conducted by reputable organizations like the CDC and vaccine manufacturers.
Main Points Highlighted by Aaron Siri:
Aaron Siri's presentation challenges the status quo surrounding vaccine mandates, providing a critical perspective on their impact and effectiveness. By bringing attention to the downsides of certain vaccines, Siri encourages a nuanced dialogue that considers both individual freedoms and public health. As the debate on vaccine mandates continues, Siri's insights contribute valuable considerations for policymakers and the public alike.
Main Points Highlighted by Aaron Siri:
- Oppression and Illiberality of Mandates: Siri asserted that mandates are inherently oppressive and illiberal, challenging the very notion of individual freedom. He argued that the ability to refuse any product, including vaccines, is essential for maintaining personal liberties.
- Vaccine Mandates Premise: The central argument for mandating vaccines often revolves around preventing the transmission of infections. Siri pointed out that several vaccines, such as those required for school entry, aim to address diseases like Hepatitis B, Polio, Measles, Mumps, Rubella, Varicella, Diphtheria, Tetanus, and Pertussis.
- Downsides of Vaccines: Siri highlighted various drawbacks supported by historical evidence and scientific studies:
- Some vaccines decrease symptoms but still allow for contagion (e.g., pertussis).
- Waning long-term effectiveness has been observed in certain vaccines (e.g., Meningococcal).
- Certain vaccines do not contribute to herd immunity (e.g., diphtheria).
- The phenomenon of linked epitope suppression can compromise immune responses (e.g., pertussis).
- Vaccines may protect individuals from disease but not necessarily from transmission (e.g., polio).
- Limited effectiveness in preventing population-wide protection (e.g., Meningococcal).
- Increased transmission in the acute phase following vaccination (e.g., chickenpox).
- Mandating vaccines for diseases with low lethality rates may be unwarranted (e.g., MMR).
Aaron Siri's presentation challenges the status quo surrounding vaccine mandates, providing a critical perspective on their impact and effectiveness. By bringing attention to the downsides of certain vaccines, Siri encourages a nuanced dialogue that considers both individual freedoms and public health. As the debate on vaccine mandates continues, Siri's insights contribute valuable considerations for policymakers and the public alike.
Greatest invention of modern medicine?
Some may consider vaccines the greatest invention of modern medicine, but are they completely guaranteed to be totally effective and safe? Before delving into mis- and disinformation surrounding vaccines, it is worth wondering if some of these contagious diseases require a vaccine. Some diseases, like measles, mumps and rubella, can be easily managed with home treatments. However, there are also some diseases, such as meningitis, the are potentially very serious. Since 2007, the Centers for Disease Control and Prevention recommends vaccinations for up to 11 different disease by the time a child is 6 years old (Centers For Disease Control and Prevention, 2007).
Unveiling Concerns Over the Expanding Childhood Vaccine Schedule
In the realm of pediatric healthcare, vaccinations have long been regarded as a cornerstone of disease prevention. However, as the recommended childhood vaccine schedule in the United States has grown substantially over the years, concerns have emerged about potential consequences for children's health. As an alarming increase in the number of recommended vaccines has trended upward, as has the surge in chronic diseases among children, and the complex interplay of factors contributing to this health landscape.
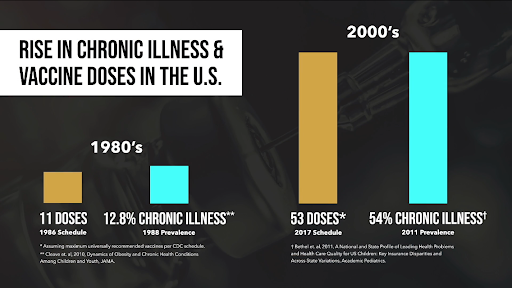
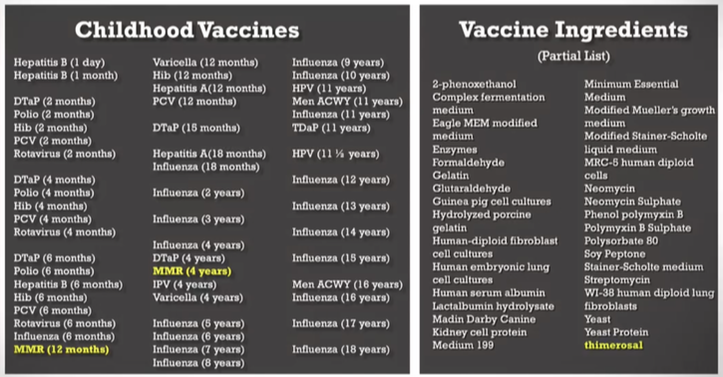
Over the past few decades, the childhood vaccine schedule recommended by the Centers for Disease Control and Prevention (CDC) has undergone a significant transformation. In 1986, children (0-19 y.o.) were recommended to receive 12 shots covering 25 antigens and protecting against 8 diseases. Fast forward to 2019, and the schedule expanded to 54 shots, encompassing 70 antigens and targeting 16 diseases, excluding COVID-19 injections (not technically a vaccine). This drastic increase in the number of vaccines raises questions about its potential impact on children's health.
Over the past few decades, the childhood vaccine schedule recommended by the Centers for Disease Control and Prevention (CDC) has undergone a significant transformation. In 1986, children (0-19 y.o.) were recommended to receive 12 shots covering 25 antigens and protecting against 8 diseases. Fast forward to 2019, and the schedule expanded to 54 shots, encompassing 70 antigens and targeting 16 diseases, excluding COVID-19 injections (not technically a vaccine). This drastic increase in the number of vaccines raises questions about its potential impact on children's health.
Concurrently, there has been a significant rise in chronic diseases among children. In the 1980s, approximately 12.8% of children were reported to have chronic health conditions. By 2006, this percentage surged to a staggering 54%. Notably, regulatory health agencies have not conducted a follow-up study to comprehensively investigate the relationship between the expanding vaccine schedule and the rise in chronic diseases. Dr. Bernadine Healy, the former director of the National Institutes of Health, acknowledged this gap and expressed concerns that researchers might be reluctant to explore potential issues with vaccines.
While acknowledging that vaccines are just one factor in the complex web of contributors to children's health, there is a growing body of concern that vaccines may play a role in the increase of chronic diseases. Vaccines are designed to interact with the immune, neurological, and endocrine systems. The intricate nature of these interactions raises questions about unintended consequences, particularly when considering the cumulative impact of multiple vaccines administered in a condensed timeframe.
While acknowledging that vaccines are just one factor in the complex web of contributors to children's health, there is a growing body of concern that vaccines may play a role in the increase of chronic diseases. Vaccines are designed to interact with the immune, neurological, and endocrine systems. The intricate nature of these interactions raises questions about unintended consequences, particularly when considering the cumulative impact of multiple vaccines administered in a condensed timeframe.
In addition to vaccines, it's essential to recognize the simultaneous increase in exposure to environmental stressors. Pesticides, heavy metals, endocrine disruptors found in plastics, petrochemicals, and air and water pollution contribute to a complex milieu affecting children's health. Understanding the multifaceted nature of these stressors is crucial for developing comprehensive public health strategies.
As concerns about the childhood vaccine schedule and the surge in chronic diseases persist, it becomes imperative to foster informed decision-making. A balanced and transparent dialogue involving healthcare professionals, researchers, and parents is crucial. Rigorous, independent studies are needed to comprehensively examine the potential long-term effects of an expanded vaccine schedule.
The confluence of an expanding childhood vaccine schedule and the rise in chronic diseases underscores the need for a nuanced, evidence-based approach to pediatric healthcare. Acknowledging the complexity of the issue and addressing concerns through transparent research and open dialogue will contribute to a more informed and responsible approach to safeguarding the health of our children.
As concerns about the childhood vaccine schedule and the surge in chronic diseases persist, it becomes imperative to foster informed decision-making. A balanced and transparent dialogue involving healthcare professionals, researchers, and parents is crucial. Rigorous, independent studies are needed to comprehensively examine the potential long-term effects of an expanded vaccine schedule.
The confluence of an expanding childhood vaccine schedule and the rise in chronic diseases underscores the need for a nuanced, evidence-based approach to pediatric healthcare. Acknowledging the complexity of the issue and addressing concerns through transparent research and open dialogue will contribute to a more informed and responsible approach to safeguarding the health of our children.
Link To Autism
In a revealing interview with investigative correspondent Sharyl Attkisson for CBS News, Dr. Bernadine Healy, a former director of the National Institutes of Health (NIH) and the first woman to head the Red Cross, shed light on a controversial aspect of the vaccines and autism debate. Driven by a commitment to scientific inquiry, Healy embarked on a journey to investigate the potential association between vaccines and autism, uncovering surprising revelations that challenged the prevailing narrative.
Healy's research led her to credible, published, and peer-reviewed scientific studies that suggested a potential association between vaccines and autism. This discovery contradicted the prevailing narrative within her professional circles.
A significant revelation was the absence of basic research conducted by the government to address the question of a link between vaccines and autism. Healy expressed surprise at the oversight, raising questions about the intention behind avoiding crucial research that could provide clarity on the issue.
Healy voiced a belief that the government and medical establishment might be intentionally avoiding the question due to a fear of the answer. The apprehension, as she saw it, stemmed from the perception of vaccines as an all-or-nothing proposition.
Healy highlighted the flawed perception that vaccines must be universally administered simultaneously, using the same vaccines. This perspective, she argued, neglects the possibility of individualized approaches to vaccination and may contribute to hesitancy in exploring potential links to autism.
Dr. Bernadine Healy's insights into the vaccines and autism debate bring forth crucial questions about the extent of research, transparency, and fear surrounding this contentious issue. As the conversation continues, Healy's perspective challenges the conventional narrative and underscores the importance of thorough, unbiased scientific exploration for the benefit of public health.
Healy's research led her to credible, published, and peer-reviewed scientific studies that suggested a potential association between vaccines and autism. This discovery contradicted the prevailing narrative within her professional circles.
A significant revelation was the absence of basic research conducted by the government to address the question of a link between vaccines and autism. Healy expressed surprise at the oversight, raising questions about the intention behind avoiding crucial research that could provide clarity on the issue.
Healy voiced a belief that the government and medical establishment might be intentionally avoiding the question due to a fear of the answer. The apprehension, as she saw it, stemmed from the perception of vaccines as an all-or-nothing proposition.
Healy highlighted the flawed perception that vaccines must be universally administered simultaneously, using the same vaccines. This perspective, she argued, neglects the possibility of individualized approaches to vaccination and may contribute to hesitancy in exploring potential links to autism.
Dr. Bernadine Healy's insights into the vaccines and autism debate bring forth crucial questions about the extent of research, transparency, and fear surrounding this contentious issue. As the conversation continues, Healy's perspective challenges the conventional narrative and underscores the importance of thorough, unbiased scientific exploration for the benefit of public health.
As to Healy's discovery, autism has in fact been linked to the MMR vaccine. Many researches have discovered the MMR vaccine to be a cause of behavioral disorders. An reanalysis of CDC data concluded that African American males receiving the MMR vaccine prior to 24 months of age or 36 months of age are more likely to receive a autism diagnosis (Hooker, 2014). The major concern is vaccines that include thimerosal, a toxic preservative that contains mercury which can damage the brain, especially among children (Geier et al., 2013). Researchers concluded stating that mercury may cause or contribute to the development of autism spectrum disorder (Kern et al., 2012). While vaccinations have progressively removing this ingredient over time, thimerosal is currently present in flu vaccines (CDC, 2016).
What is autism?
Autism Spectrum Disorder (ASD) remains a complex neurodevelopmental presentation, characterized by a spectrum of impairments that affect social interaction, communication, and behavioral patterns. Beyond behavioral definitions, recent research has shed light on potential underlying causes, emphasizing the intertwined roles of inflammation, oxidative stress, and intriguing connections to gastrointestinal health and maternal diet and lifestyle. In this exploration, we delve into the characteristics of ASD and the emerging understanding of its multifaceted origins.
Characteristics of ASD:
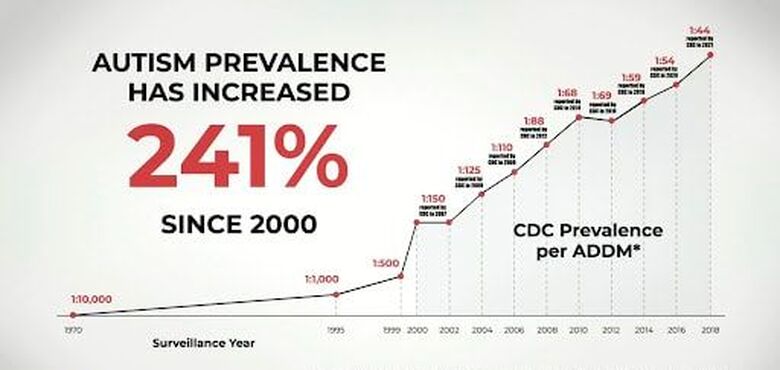
The rise in autism rates from 1 in 10,000 in the 1970s to 1 in 44 in 2021 sparks contemplation. Given the identified underlying causes of ASD, the surge may be attributed to a complex interplay of factors since the 1970s. Environmental contributors, including vaccines, pesticides, petrochemicals, air pollution, and endocrine disruptors found in plastics, likely contribute to the manifestation of autism.
Characteristics of ASD:
- Behavioral Impairments: ASD is defined by a spectrum of qualitative impairments, including challenges in social interaction, communication, and restrictive or stereotyped behaviors. The diverse nature of these impairments contributes to the uniqueness of each individual's experience within the spectrum.
- Psychiatric Diagnosis: ASD is diagnosed based on psychiatric evaluations that consider a range of behavioral criteria. The diagnosis relies on identifying patterns of behavior, interests, and activities that distinguish individuals with ASD from neurotypical peers.
- Neuroinflammation and Encephalitis: Ongoing neuroinflammation or encephalitis in various brain regions has been associated with ASD. Elevated proinflammatory cytokine profiles are observed, indicating a heightened immune response that may influence neurodevelopmental processes.
- Comorbid Medical Conditions: Children with ASD often experience co-morbid medical conditions, with gastrointestinal (GI) disorders being prevalent. The interplay between GI health and ASD suggests a broader systemic impact beyond neurological manifestations.
- Intestinal Microbiota Disparities: Research indicates that children with ASD possess different intestinal microbiota populations compared to neurotypical children. The intricate gut-brain axis is implicated, emphasizing the potential role of the microbiome in ASD pathogenesis.
- Maternal Diet Influence: Animal studies demonstrate a link between a maternal high-fat diet (HFD), gut microbiota dysbiosis, and changes in central neurobiology leading to abnormal social behaviors in offspring. This connection between maternal diet, dysbiosis, and neurodevelopmental disorders raises intriguing possibilities.
- Microbiome Manipulation as Therapeutic Option: Animal research suggests that altering the microbiome may correct brain and behavioral defects induced by dysbiosis, presenting the microbiota as a potential underlying cause of central nervous system dysfunction in ASD. This opens avenues for novel therapeutic options.
The rise in autism rates from 1 in 10,000 in the 1970s to 1 in 44 in 2021 sparks contemplation. Given the identified underlying causes of ASD, the surge may be attributed to a complex interplay of factors since the 1970s. Environmental contributors, including vaccines, pesticides, petrochemicals, air pollution, and endocrine disruptors found in plastics, likely contribute to the manifestation of autism.
The evolving understanding of ASD encompasses not only its behavioral characteristics but also delves into the intricate interplay of inflammation, oxidative stress, and the gut microbiome. Recognizing the systemic nature of ASD offers hope for targeted interventions and underscores the importance of holistic approaches to unraveling the enigma of this complex neurodevelopmental disorder.
Reports on Thimerosal
Growing numbers of Americans are refusing to vaccinate their children because they think vaccines are causing autism. But it's not the vaccines that appear to be one cause of neurological disorders, it's something else. Now, a senior CDC vaccine safety scientist has invoked the protection of the Federal Whistleblower Statute, and is claiming that the CDC knew that thimerosal was unsafe, but pressured him to publish studies claiming otherwise.
Know Your vaccines - is the cure worse than the disease
In discussions surrounding vaccines targeting various pathogens, including the seasonal flu, H1N1, among others, investigative journalist James Corbett from The Corbett Report asks whether the cure is worse than the disease. In this podcast he highlights various important points, including a obvious psychological operation, featuring a CBS broadcast garnered attention as an anchorwoman received the flu shot live on air. This move was met with scrutiny, prompting an exploration into the broader landscape of vaccine controversies. From the swine flu vaccine's approval to concerns about side effects, a series of revelations has brought to light the multifaceted discourse on vaccination safety and ethics.
CBS attempts to encourage viewers to take the seasonal flu vaccine, adding to the anticipation for the release of the swine flu vaccine. However, concerns arise regarding potential side effects, leading some medical professionals, as revealed by Wayne Madsen on RussiaToday.com, to express reluctance towards taking the swine flu vaccine.
The swine flu vaccine has received approval in the United States, but questions linger about the testing of these vaccines on the target population. Highlighting this concern, reports indicate that vaccine trials for high-risk groups are yet to be completed.
Allegations of vaccine-promoting doctors being funded by vaccine manufacturers raise ethical questions. Harvard Medical School comes under scrutiny for potential ties with Big Pharma, contributing to concerns about the impartiality of vaccine endorsements.
A cascade of research suggests that vaccines may have potentially deadly consequences, challenging the narrative of their universal safety. From questionable additives to the emergency use of unapproved substances in swine flu shots, the safety of vaccines becomes a focal point of debate.
Controversy surrounds the use of adjuvants, with the HHS reportedly paying substantial amounts to pharmaceutical giants GSK and Novartis for adjuvants in their swine flu vaccines. The potential health hazards of vaccine adjuvants, especially aluminum, raise alarm bells.
Scientific research links squalene to health issues, drawing parallels to Gulf War Syndrome. As new squalene adjuvants are introduced into swine flu shots, questions arise about their safety and potential implications.
Canada's Bill C-6 comes into focus, outlining the possibility of government-backed mass vaccinations. This legislative move stirs concerns about individual autonomy and the potential for mandatory vaccination programs.
The ongoing debate surrounding flu shots brings to the forefront a myriad of concerns, from financial ties influencing medical endorsements to the safety of vaccine additives. As individuals and governments grapple with the complexities of vaccination, the need for transparency, rigorous testing, and ethical considerations becomes increasingly evident in navigating the path forward.
CBS attempts to encourage viewers to take the seasonal flu vaccine, adding to the anticipation for the release of the swine flu vaccine. However, concerns arise regarding potential side effects, leading some medical professionals, as revealed by Wayne Madsen on RussiaToday.com, to express reluctance towards taking the swine flu vaccine.
The swine flu vaccine has received approval in the United States, but questions linger about the testing of these vaccines on the target population. Highlighting this concern, reports indicate that vaccine trials for high-risk groups are yet to be completed.
Allegations of vaccine-promoting doctors being funded by vaccine manufacturers raise ethical questions. Harvard Medical School comes under scrutiny for potential ties with Big Pharma, contributing to concerns about the impartiality of vaccine endorsements.
A cascade of research suggests that vaccines may have potentially deadly consequences, challenging the narrative of their universal safety. From questionable additives to the emergency use of unapproved substances in swine flu shots, the safety of vaccines becomes a focal point of debate.
Controversy surrounds the use of adjuvants, with the HHS reportedly paying substantial amounts to pharmaceutical giants GSK and Novartis for adjuvants in their swine flu vaccines. The potential health hazards of vaccine adjuvants, especially aluminum, raise alarm bells.
Scientific research links squalene to health issues, drawing parallels to Gulf War Syndrome. As new squalene adjuvants are introduced into swine flu shots, questions arise about their safety and potential implications.
Canada's Bill C-6 comes into focus, outlining the possibility of government-backed mass vaccinations. This legislative move stirs concerns about individual autonomy and the potential for mandatory vaccination programs.
The ongoing debate surrounding flu shots brings to the forefront a myriad of concerns, from financial ties influencing medical endorsements to the safety of vaccine additives. As individuals and governments grapple with the complexities of vaccination, the need for transparency, rigorous testing, and ethical considerations becomes increasingly evident in navigating the path forward.
vaccines as silent weapons
In a revealing podcast by James Corbett, the world of vaccines is exposed as a complex web of hidden agendas, experimental initiatives, and potential dangers. Corbett delves into the darker side of vaccinations, suggesting their role as silent weapons in a broader quiet war of soft kill eugenics.
The podcast commences with an examination of the fundamental question: What is a vaccine? Corbett provides insights into the basics of vaccine functionality, setting the stage for a critical analysis of the controversial aspects surrounding these medical interventions.
A critical inquiry into the contents of flu shots prompts viewers to question the transparency and scientific basis behind these widely administered vaccinations. Corbett challenges the conventional narrative, raising concerns about the substances included in flu shots.
The podcast takes a dramatic turn with a confession from Dr. Maurice Hilleman, associated with Merck. This revelation exposes the presence of cancer and other viruses in vaccines, emphasizing the potential risks associated with these inoculations.
Corbett unveils the involvement of influential figures like Rockefeller and Gates in experimental vaccine initiatives. The presentation of the David Rockefeller Bridging Leadership Award to the Gates family raises suspicions about their roles in population reduction strategies through vaccination programs.
Cynthia, a whistleblower connected to the Gates Foundation, provides startling insights into the inner workings of vaccine agendas. The podcast shares excerpts from her interview, shedding light on potential hidden motives behind vaccine initiatives.
Corbett explores Gates-funded projects involving "flying syringe" mosquitos, introducing unconventional ideas that receive financial backing. This segment emphasizes the intricate interests and motivations behind vaccine research.
The controversial Gardasil vaccine takes center stage as Mike Adams of NaturalNews exposes potential risks and scams associated with this medical intervention. Corbett highlights concerns about the safety and efficacy of Gardasil, challenging established narratives.
Adding a touch of humor, the podcast discusses the unpredictable nature of choosing appropriate strains for flu shots each year. Corbett questions the scientific basis behind flu vaccine development, encouraging viewers to reevaluate their perceptions.
Corbett explores the ethical dilemma faced by doctors in addressing patient skepticism regarding flu vaccines. The podcast provides insights into strategies employed to encourage vaccine compliance, raising questions about informed consent.
The exploration concludes with a reference to Alan Watt's concept of silent weapons. Corbett emphasizes the potential dangers and covert intentions behind vaccination programs, leaving listeners to ponder the implications of soft kill eugenics in the realm of public health.
James Corbett's podcast serves as a thought-provoking journey, uncovering hidden facets of the vaccine landscape. It challenges preconceptions, raises awareness about potential risks, and encourages a critical examination of the broader implications of vaccine initiatives.
The podcast commences with an examination of the fundamental question: What is a vaccine? Corbett provides insights into the basics of vaccine functionality, setting the stage for a critical analysis of the controversial aspects surrounding these medical interventions.
A critical inquiry into the contents of flu shots prompts viewers to question the transparency and scientific basis behind these widely administered vaccinations. Corbett challenges the conventional narrative, raising concerns about the substances included in flu shots.
The podcast takes a dramatic turn with a confession from Dr. Maurice Hilleman, associated with Merck. This revelation exposes the presence of cancer and other viruses in vaccines, emphasizing the potential risks associated with these inoculations.
Corbett unveils the involvement of influential figures like Rockefeller and Gates in experimental vaccine initiatives. The presentation of the David Rockefeller Bridging Leadership Award to the Gates family raises suspicions about their roles in population reduction strategies through vaccination programs.
Cynthia, a whistleblower connected to the Gates Foundation, provides startling insights into the inner workings of vaccine agendas. The podcast shares excerpts from her interview, shedding light on potential hidden motives behind vaccine initiatives.
Corbett explores Gates-funded projects involving "flying syringe" mosquitos, introducing unconventional ideas that receive financial backing. This segment emphasizes the intricate interests and motivations behind vaccine research.
The controversial Gardasil vaccine takes center stage as Mike Adams of NaturalNews exposes potential risks and scams associated with this medical intervention. Corbett highlights concerns about the safety and efficacy of Gardasil, challenging established narratives.
Adding a touch of humor, the podcast discusses the unpredictable nature of choosing appropriate strains for flu shots each year. Corbett questions the scientific basis behind flu vaccine development, encouraging viewers to reevaluate their perceptions.
Corbett explores the ethical dilemma faced by doctors in addressing patient skepticism regarding flu vaccines. The podcast provides insights into strategies employed to encourage vaccine compliance, raising questions about informed consent.
The exploration concludes with a reference to Alan Watt's concept of silent weapons. Corbett emphasizes the potential dangers and covert intentions behind vaccination programs, leaving listeners to ponder the implications of soft kill eugenics in the realm of public health.
James Corbett's podcast serves as a thought-provoking journey, uncovering hidden facets of the vaccine landscape. It challenges preconceptions, raises awareness about potential risks, and encourages a critical examination of the broader implications of vaccine initiatives.
Follow the Money
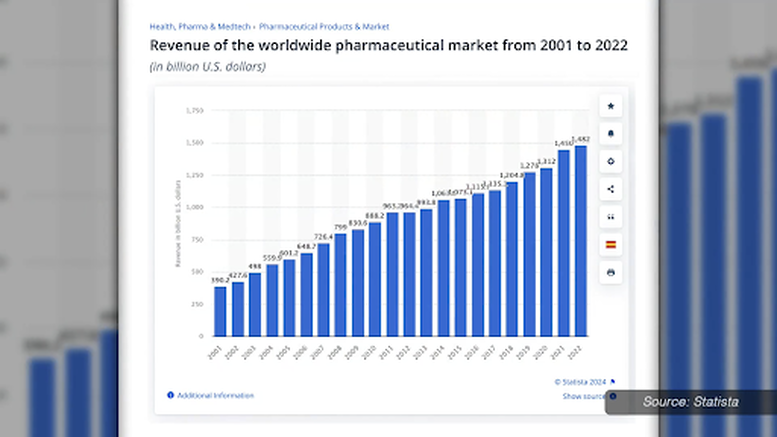
The landscape of vaccinations has transformed into a formidable industry, with financial figures painting a staggering picture of its growth and influence. According to Grand View Research (2017), vaccinations are projected to be a colossal $77 billion industry by 2024. This financial surge is reflective of broader trends within the pharmaceutical market, which reached a staggering $1,482 billion in revenue worldwide in 2022. As the monetary stakes continue to rise, the influence wielded by pharmaceutical giants in shaping medical narratives becomes increasingly evident, giving rise to concerns about the impartiality of the healthcare system.
Pharmaceutical companies involved in vaccine production leverage their substantial financial resources to exert influence over various sectors, creating a web of interconnected interests. This influence extends to governments, the medical establishment, and physicians, shaping policies and practices that align with their financial interests. The result is a scenario where those responsible for creating and marketing vaccines are deeply involved in steering the narrative surrounding their necessity.
The analogy of the fox guarding the hen house becomes all too apt in the context of vaccine manufacturers shaping the discourse around immunization. The inherent conflict of interest arises when entities with a direct financial stake in vaccine sales play a significant role in guiding healthcare policies. This influence poses a potential risk, as financial motivations may overshadow the pursuit of unbiased, evidence-based medical practices.
The analogy of the fox guarding the hen house becomes all too apt in the context of vaccine manufacturers shaping the discourse around immunization. The inherent conflict of interest arises when entities with a direct financial stake in vaccine sales play a significant role in guiding healthcare policies. This influence poses a potential risk, as financial motivations may overshadow the pursuit of unbiased, evidence-based medical practices.
The financial prowess of pharmaceutical companies enables them to engage in extensive lobbying efforts, marketing campaigns, and partnerships that strategically position vaccines as indispensable tools for preventing diseases. This concerted effort often leads to the muddying of objective decision-making processes within the medical community, as physicians may be inadvertently influenced by misinformation or skewed narratives.
As the financial dimensions of the vaccine industry continue to expand, the need for transparency and safeguards against undue influence becomes imperative. Navigating this complex terrain requires a critical examination of the relationships between pharmaceutical companies, government bodies, and healthcare professionals. It calls for a reassessment of the checks and balances in place to ensure that public health decisions are driven by scientific evidence rather than financial considerations.
The financial trajectory of the vaccine industry reveals a lucrative landscape, raising questions about the potential conflicts of interest and the sway of money over medical decision-making. Recognizing the power dynamics at play is crucial for fostering an environment where public health policies are guided by a commitment to unbiased science and the wellbeing of communities, rather than the financial interests of a select few.
As the financial dimensions of the vaccine industry continue to expand, the need for transparency and safeguards against undue influence becomes imperative. Navigating this complex terrain requires a critical examination of the relationships between pharmaceutical companies, government bodies, and healthcare professionals. It calls for a reassessment of the checks and balances in place to ensure that public health decisions are driven by scientific evidence rather than financial considerations.
The financial trajectory of the vaccine industry reveals a lucrative landscape, raising questions about the potential conflicts of interest and the sway of money over medical decision-making. Recognizing the power dynamics at play is crucial for fostering an environment where public health policies are guided by a commitment to unbiased science and the wellbeing of communities, rather than the financial interests of a select few.
More Information
Vaccines have been a focused topic of Mindful Wellness for years. The data suggesting vaccines are neither safe or effective for everyone is overwhelming, given that you as the reader are able to put your confirmation bias aside and look at the information critically with an open-mind.
References
Centers for Disease Control and Prevention [CDC]. "2007 Childhood & Adolescent Immunization Schedules." National Immunization Program. www.cdc.gov/nip/recs/child-schedule.htm#printable (accessed February 1, 2017)
CDC. (2016, August 3). Making the vaccine decision. Retrieved February 4, 2017, from Centers for Disease Control and Prevention, https://www.cdc.gov/vaccines/parents/vaccine-decision/index.html
Geier, D. A., Hooker, B. S., Kern, J. K., King, P. G., Sykes, L. K., Geier, M. R., … Spring, S. (2013). A two-phase study evaluating the relationship between Thimerosal-containing vaccine administration and the risk for an autism spectrum disorder diagnosis in the United States. Translational Neurodegeneration, 2(1), 25. doi:10.1186/2047-9158-2-25
Grand View Research. (2017). Vaccine market size projected to reach $77.5 Billion by 2024. Retrieved February 2, 2017, from https://www.grandviewresearch.com/press-release/global-vaccine-market
Hooker, B. S. (2014). Measles-mumps-rubella vaccination timing and autism among young african american boys: A reanalysis of CDC data. Translational Neurodegeneration, 3(1), 16. doi:10.1186/2047-9158-3-16
Kern, J., Geier, D., Audhya, T., King, P., Sykes, L., & Geier (2012). Evidence of parallels between mercury intoxication and the brain pathology in autism. Acta neurobiologiae experimentalis., 72(2), 113–53. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22810216
Bichel, “Post-vaccinial Lymphadenitis Developing into Hodgkin’s Disease”, Acta Med Scand, 1976, Vol 199, p523-525.
Stewart, AM, et al, “Aetiology of Childhood Leukaemia”, Lancet, 16 Oct, 1965, 2:789-790. [Listed under Vaccine Adverse Reactions.]
Glathe, H et al, “Evidence of Tumorigenic Activity of Candidate Cell Substrate in Vaccine Production by the Use of Anti-Lymphocyte Serum”, Development Biol Std, 1977, 34:145-148.
Bolognesi, DP, “Potential Leukemia Virus Subunit Vaccines: Discussion”, Can Research, Feb 1976, 36(2 pt 2):655-656.
Colon, VF, et al, “Vaccinia Necrosum as a Clue to Lymphatic Lymphoma”, Geriatrics, Dec 1968, 23:81-82.
Park-Dincsoy, H et al, “Lymphoid Depletion in a case of Vaccinia Gangrenosa”, Laval Med, Jan 1968, 39:24-26.
Hugoson, G et al, “The Occurrence of Bovine Leukosis Following the Introduction of Babesiosis Vaccination”, Bibl Haemat, 1968, 30:157-161.
Hartstock, , “”Post-vaccinial Lymphadenitis: Hyperplasia of Lymphoid Tissue That Simulates Malignant Lymphomas”, Apr 1968, Cancer, 21(4):632-649.
Allerberger, F, “An Outbreak of Suppurative Lymphadenitis Connected with BCG Vaccination in Austria- 1990/1991,” Am Rev Respir Disorder, Aug 1991, 144(2) 469.
Omokoku B, Castells S, “Post-DPT inoculation cervical lymphadenitis in children.” N Y State J Med 1981 Oct;81(11):1667-1668.
Knuutila, S et al, “An Increased Frequency of Chromosomal Changes and SCE’s in Cultured Lymphocytes of 12 Subjects Vaccinated Against Smallpox,” Hum Genet, 1978 Feb 23; 41(1):89-96.
Cherkeziia, SE, et al, “Disorders in the Murine Chromosome Apparatus Induced By Immunization with a Complex of Anti-viral Vaccines,” Vopr Virusol, 1979 Sept Oct, (5):547-550.
Romanov, V A, et al, "Role of Auto-immune Processes in the Pathogenesis of Post-Vaccinal Lesions of the Nervous System", Oct 1977, Zh Mikrobiol Epidemiol Immunobiol, 10:80-83.
Grachev, V P, et al, "Formation of Auto-antibodies in Laboratory Animals After Inoculation of Viruses With Different Virulence. I. Results of Studies ..., July 1973, Acta Virol (Praha), 17:319-326.
Movsesiants, AA, et al, "Experimental Study of the Ability of Different Strains of Vaccinia Virus to Induce Auto-Antibody Formation", Vopr Virusol, May-Jun 1975; (3):297-302.
Sinaniotis, et al, "Diabetes Mellitus after Mumps Vaccination", Arc Dis Child, 1975, 50:749.66
Polster, H, "Diabetes insipidus after Smallpox vaccination", Z Aerztl Fortbild (Jena), 1 Apr 1966, 60:429-432.
Patan, "Postvaccinal Severe Diabetes Mellitus", Ter Arkh, Jul 1968, 40:117-118.
Classen, JB, MD, "The Timing of Immunization Affects The Development of Diabetes in Rodents", Autoimmunity, 1996, 24:137-145.
Classen JB, "The diabetes epidemic and the hepatitis B vaccines," N Z Med J, 109(1030):366 1996 Sep 27. [letter]
Classen JB, “Childhood immunisation and diabetes mellitus,” N Z Med J, 109(1022):195 1996 May 24 [letter]
Poutasi K, ” Immunisation and diabetes,” N Z Med J 1996 Jul 26;109(1026):283. [letter; comment] Other Articles Linking Diabetes to Vaccines:
Dokheel, T M, “An Epidemic of Childhood Diabetes in the United States? Evidence from ….”, Diabetes Care, 1993, 16:1606-1611.
Parent ME, et al, “Bacille Calmette-Guerin vaccination and incidence of IDDM in Montreal, Canada,” Diabetes Care 1997 May; 20(5):767-772.
House DV, Winter WE, “Autoimmune diabetes. The role of auto-antibody markers in the prediction and prevention of insulin-dependent diabetes mellitus,” Clin Lab Med 1997 Sep; 17(3):499-545.
Zeigler, M et al , “[Autoantibodies in type 1 diabetes mellitus]” Z Arztl Fortbild (Jena). 1994 Aug; 88(7-8):561-5
Bondarev, VN et al, “The Changes of the Nervous System in Children After Vaccination”, Pediatria, Jun 1969; 48:20-24.
Ehrengut W, “Central nervous sequelae of vaccinations,” Lancet 1986 May 31;1(8492):1275-1276.
Provvidenza, G et al, [On a Case of Benign Acute Cerebellar Ataxia in Childhood], Arch Ital Sci Med Trop, 43:189-194, Apr 1962.
Katsilambros, L, “[The Phenomenom of Apathy in Man and Animals After the Injection of Viruses in Very High Doses. Clinical Data]“, Rev Med Moyen Orient, 20:539-546, Nov – Dec 1963.
Eggers, C, “Autistic Syndrome (Kanner) And Vaccinations against Smallpox”, Klin Paediatr, Mar 1976, 188(2):172-180.
Kiln MR, “Autism, inflammatory bowel disease, and MMR vaccine.” Lancet 1998 May 2;351(9112):1358.
Selway, “MMR vaccination and autism 1998. Medical practitioners need to give more than reassurance.” BMJ 1998 Jun 13;316(7147):1824.
Nicoll A, Elliman D, Ross E, “MMR vaccination and autism 1998,” MJ 1998 Mar 7;316(7133):715-716.
Lindley K J, Milla PJ, “Autism, inflammatory bowel disease, and MMR vaccine.”Lancet 1998 Mar 21;351(9106):907-908.
Bedford H, et al, “Autism, inflammatory bowel disease, and MMR vaccine.” Lancet 1998 Mar 21;351(9106):907.
Vijendra K. Singh, Sheren X. Lin, and Victor C. Yang, “Serological Association of Measles Virus and Human Herpesvirus-6 with Brain Autoantibodies in Autism,” Clinical Immunology and Immunopathology, Oct 1998, Vol. 89, No. 1, p 105-108.
Herroelen, L et al, "Central-Nervous-System Demyelination After Immunization with Recombinant Hepatitis B Vaccine", Lancet, Nov 9, 1991, 338(8776):1174-1175.
Kaplanski G, Retornaz F, Durand J, Soubeyrand J, "Central nervous system demyelination after vaccination against hepatitis B and HLA haplotype." J Neurol Neurosurg Psychiatry 1995 Jun; 58(6):758-759.
Matyszak MK, Perry VH, "Demyelination in the central nervous system following a delayed-type hypersensitivity response to bacillus Calmette-Guerin." Neuroscience 1995 Feb;64(4):967-977.
Tornatore CS, Richert JR, "CNS demyelination associated with diploid cell rabies vaccine." Lancet 1990 Jun 2;335(8701):1346-1347.
Adams, JM et al, "Neuromyelitis Optica: Severe Demyelination Occurring Years After Primary Smallpox Vaccinations", Rev Roum Neurol, 1973, 10:227-231.
Hirtz DG, Nelson KB, Ellenberg J H, "Seizures following childhood immunizations", Pediatr 1983 Jan; 102(1):14-18.
Cherry JD, Holtzman AE, Shields WD, Buch D, Nielsen, "Pertussis immunization and characteristics related to first seizures in infants and children,"J Pediatr 1993 Jun;122(6):900-903.
Coplan J, "Seizures following immunizations," J Pediatr 1983 Sep;103(3):496.
Barkin RM, Jabhour JT, Samuelson J S, "Immunizations, seizures, and subsequent evaluation," JAMA 1987 Jul 10;258(2):201.
Griffin MR, et al, "Risk of seizures after measles-mumps-rubella immunization," Pediatrics 1991 Nov;88(5):881-885.
Griffin MR, et al, "Risk of seizures and encephalopathy after immunization with the diphtheria-tetanus-pertussis vaccine," JAMA 1990 Mar 23-30;263(12):1641-1645.
Cizewska S, Huber Z, Sluzewski W, "[Prophylactic inoculations and seizure activity in the EEG],” Neurol Neurochir Pol 1981 Sep-Dec;15(5-6):553-557. [Article in Polish]
Huttenlocher PR, Hapke RJ, “A follow-up study of intractable seizures in childhood.” Ann Neurol 1990 Nov; 28(5):699-705.
Blumberg DA, “Severe reactions associated with diphtheria-tetanus-pertussis vaccine: detailed study of children with seizures, hypotonic-hypo-responsive episodes, high fevers, and persistent crying.”Pediatrics 1993 Jun; 91(6):1158-1165.
Prensky AL, et al, “History of convulsions and use of pertussis vaccine,” J Pediatr 1985 Aug; 107(2):244-255.
Baraff LJ, “Infants and children with convulsions and hypotonic-hypo-responsive episodes following diphtheria-tetanus-pertussis immunization: follow-up evaluation,” Pediatrics 1988 Jun; 81(6):789-794.
Jacobson V, “Relationship of pertussis immunization to the onset of epilepsy, febrile convulsions and central nervous system infections: a retrospective epidemiologic study,” Tokai J Exp Clin Med 1988;13 Suppl: 137-142.
Cupic V,et al, “[Role of DTP vaccine in the convulsive syndromes in children],” Lijec Vjesn 1978 Jun; 100(6):345-348. [Article in Serbo-Croatian (Roman)]
Pokrovskaia NIa, “[Convulsive syndrome in DPT vaccination (a clinico-experimental study)],” Pediatriia 1983 May;(5):37-39. [Article in Russian]
Ballerini, Ricci, B, et al, “On Neurological Complications of Vaccination, With Special Reference to Epileptic Syndromes,” Riv Neurol, Jul-Aug 1973, 43:254-258.
Wolf SM, Forsythe A, “Epilepsy and mental retardation following febrile seizures in childhood,” Acta Paediatr Scand 1989 Mar;78(2):291-295.
Iwasa, S et al, “Swelling of the Brain in Mice Caused by Pertussis … Quantitative Determination and the Responsibility of the Vaccine”, Jpn J Med Sci Biol, 1985 , 38(2):53-65.
Mathur R, Kumari S, “Bulging fontanel following triple vaccine.” Indian Pediatr 1981 Jun;18(6):417-418.
Barry W, Lenney W, Hatcher G, “Bulging fontanelles in infants without meningitis.” Arch Dis Child 1989 Apr;64(4):635-636.
Shendurnikar N, “Bulging fontanel following DPT” Indian Pediatr 1986 Nov;23(11):960.
Gross TP, Milstien JB, Kuritsky JN, “Bulging fontanelle after immunization with diphtheria-tetanus-pertussis vaccine and diphtheria-tetanus vaccine.” J Pediatr 1989 Mar;114(3):423-425.
Jacob J, Mannino F, “Increased intracranial pressure after diphtheria, tetanus, and pertussis immunization.” Am J Dis Child 1979 Feb;133(2):217-218.
Dugmore, WN, “Bilateral Oedema at the Posterior Pole. Hypersensitivity Reaction to Alavac P injection.” Br J Ophthalmol, Dec 1972, 55:848-849.
Nedar P R, and Warren, R J, “Reported Neurological Disorders Following Live Measles Vaccine”, 1968, Ped, 41:997-1001.
Paradiso, G et al, “Multifocal Demyelinating Neuropathy after Tetanus Vaccine”, Medicina (B Aires), 1990, 50(1):52-54.
Landrigan, PJ, Whitte, J, “Neurologic Disorders Following Live Measles-virus Vaccination”, JAMA, Mar 26, 1973, v223(13):1459-1462.
Turnbull, H M, “Encephalomyelitis Following Vaccination”, Brit Jour Exper Path, 7:181, 1926.
Kulenkampff, M et al, “Neurological Complications of Pertussis Inoculation”, Arch Dis Child, 1974, 49:46.
Strom, J, “Further Experience of Reactions, Especially of a Cerebral Nature in Conjunction with Triple Vaccination”, Brit Med Jour, 1967, 4:320-323.
Berg, J M, “Neurological Complications of Pertussis Immunization,” Brit Med Jour, July 5,1958; p 24.
Bondarev, VN et al, “The Changes of the Nervous System in Children After Vaccination”, Pediatria, Jun 1969; 48:20-24.
Badalian, LO, “Vaccinal Lesions of the Nervous System in Children,” Vop Okhr Materin Dets, Dec 1959, 13:54-59
Lorentz, IT, et al, “Post-Vaccinal Sensory Polyneuropathy with Myoclonus”, Proc Aust Ass Neurol, 1969, 6:81-86.
Trump, R C, White, T R, “Cerebellar Ataxia Presumed Due To Live Attenuated Measles Virus Vaccine,” JAMA, 1967, 199:165-166.
Allerdist, H, “Neurological Complications Following Measles Vaccination”, Inter Symp, Brussels, 1978, Development Biol Std, Vol 43, 259-264.
Finley, K H, “Pathogenesis of Encephalitis Occurring With Vaccination, Variola and Measles, Arch Neur and Psychologist, 1938; 39:1047-1054.
Froissart, M et al, “Acute Meningoencephalitis Immediately after an Influenza Vaccination”, Lille Med, Oct 1978, 23(8):548-551.
Pokrovskaia, Nia, et al, “Neurological Complications in Children From Smallpox Vaccination”, Pediatriia, Dec 1978, (12):45-49.
Allerdist, H, “Neurological Complications Following Measles Virus Vaccination. Evaluation of the Cases seen Between 1971-1977″, Monatsschr Kinderheilkd, Jan 1979, 127(1): 23-28.
Ehrengut, W et al, “On Convulsive Reactions Following Oral vaccination Against Polio”, Klin Paediatr, May 1979, 191(3):261-270.
Naumova, R P, et al, “Encephalitis Developing After Vaccination without a Local Skin Reaction”, Vrach Delo, Jul 1979, (7):114-115.
Goswamy, BM, “Neurological Complications After Smallpox Vaccination”, J Ass Phys India, Jan 1969, 17:41-43.
Schchelkunov, SN et al, “The Role of Viruses in the Induction of Allergic Encephalomyelitis,” Dokl Akad Nauk SSSR, 1990,315(1):252-255.
Walker AM, “Neurologic events following diphtheria-tetanus-pertussis immunization,” Pediatrics 1988 Mar;81(3):345-349.
Shields WD, et al, “Relationship of pertussis immunization to the onset of neurologic disorders: a retrospective epidemiologic study,” J Pediatr 1988 Nov; 113(5):801-805.
Wilson J, “Proceedings: Neurological complications of DPT inoculation in infancy,” Arch Dis Child 1973 Oct; 48(10):829-830.
Iakunin IuA, “[Nervous system complications in children after preventive vaccinations],” Pediatriia 1968 Nov; 47(11):19-26. [Article in Russian]
Greco D, et al, “Case-control study on encephalopathy associated with diphtheria-tetanus immunization in Campania, Italy,” Bull World Health Organ 1985;63(5):919-925.
Ehrengut W, “Bias in evaluating CNS complications following pertussis immunization.” Acta Paediatr Jpn, 1991 Aug; 33(4):421-427.
Hiner, E E, Frasch, C E, “Spectrum of Disease Due to Haemophilus Influenza Type B Occurring in Vaccinated Children”, J Infect Disorder, 1988 Aug; 158(2): 343-348.
Olin P, Romanus, V, Storsaeter, J, “Invasive Bacterial Infections During an Efficiacy Trial of Acellular Pertussis Vaccines — Implications For Future Surveilance In Pertussis Vaccine Programmes”, Tokai J Exp Clin Med, 1988; 13 Suppl: 143-144.
Storsaeter, J, et al, “Mortality and Morbidity From Invasive Bacterial Infections During a Clinical Trial of Acellular Pertussis Vaccines in Sweden”, Pediatr Infect Disorder J, 1988 Sept; 7(9):637-645.
Vadheim, CM, et al, “Effectiveness and Safety of an Haemophilus Influenzae type b Conjugate Vaccine (PRP-T) in Young Infants. Kaiser-UCLA Vaccine Study Group,” Pediartics, 1993 Aug; 92(2):272-279.
Stickl, H, “Estimation of Vaccination Damage”, Med Welt, Oct 14, 1972, 23:1495-1497.
Waters, VV, et al, “Risk Factors for Measles in a Vaccinated Population”, JAMA, Mar 27, 1991, 265(12): 1527.
Stickl, H, “Iatrogenic Immuno-suppression as a Result of Vaccination”, Fortschr Med, Mar 5, 1981, 99(9);289-292.
Nkowane, et al, “Vaccine-Associated Paralytic Poliomyelitis, US 1973 through 1984, JAMA, 1987, Vol 257:1335-1340.
Quast, et al, “Vaccine Induced Mumps-like Diseases”, nd, Int Symp on Immun, Development Bio Stand, Vol 43, p269-272.
Green, C et al, “A Case of Hepatitis Related to Etretinate Therapy and Hepatitis B Vaccine”, Dermatologica, 1991, 182(2):119-120.
Shasby, DM, et al, “Epidemic Measles in Highly Vaccinated Population”, NEJM, Mar 1977, 296(11): 585-589.
Tesovic, G et al, “Aseptic Meningitis after Measles, Mumps and Rubella Vaccine”, Lancet, Jun 12, 1993, 341(8859):1541.
Johnson, RH, et al, “Nosocomial Vaccinia Infection”, West J Med, Oct 1976, 125(4):266-270.
Malengreau, M, “Reappearance of Post-Vaccination Infection of Measles, Rubella, and Mumps. Should Adolescents be re-vaccinated?” Pedaitric, 1992;47(9):597-601 (25 ref)
Basa, SN, “Paralytic Poliomyelitis Following Inoculation With Combined DTP Prophylactic. A review of Sixteen cases with Special Reference to Immunization Schedules in Infancy”, J Indian Med Assoc, Feb 1, 1973, 60:97-99.
Landrigan, PJ et al, “Measles in Previously Vaccinated Children in Illinois”, Ill Med J, Arp 1974, 141:367-372.
NA, “Vaccine-Associated Poliomyelitis”, Med J Aust, Oct 1973, 2:795-796.
Hardy, GE, Jr, et al, “The Failure of a School Immunization Campaign to Terminate an Urban Epidemic of Measles,” Amer J Epidem, Mar 1970; 91:286-293.
Cherry, JD, et al, “A Clinical and Serologic Study of 103 Children With Measles Vaccine Failure”, J Pediatr, May 1973; 82:801-808.
Jilg, W, et al, “Inoculation Failure Following Hepatitis B Vaccination”, Dtsch Med wochenschr, 1990 Oct 12; 115(41):1514-1548.
Plotkin, SA, “Failures of Protection by Measles Vaccine,” J Pediatr, May 1973; 82:798-801.
Bolotovskii, V, et al, “Measles Incidence Among Children Properly Vaccinated Against This Infection”, ZH Mikrobiol Epidemiol Immunobiol, 1974; 00(5):32-35.
Landrigan, PJ, et al, “Measles in Previously Vaccinated Children in Illinois”, Ill Med J, Apr 1974; 141:367-372.
Strebel, P et al, “An Outbreak of Whooping Cough in a Highly Vaccinated Urban Community”, J Trop Pediatr, Mar 1991, 37(2): 71-76.
Forrest, JM, et al, “Failure of Rubella Vaccination to Prevent Congenital Rubella,”Med J Aust, 1977 Jan 15; 1(3): 77.
Jilg, W, “Unsuccessful Vaccination against Hepatitis B”, Dtsch Med Wochenschr, Nov 16, 1990, 115(46):1773.
Coles, FB, et al, “An Outbreak of Influenza A (H3N2) in a Well-Immunized Nursing home Population,” J Am ger Sociologist, Jun 1992, 40(6):589-592.
Jilg, W, et al, “Inoculation Failure following Hepatitis B Vaccination,” Dtsch Med Wochenschr, Oct 12, 1990, 115(41):1545-1548.
Hartmann, G et al, “Unsuccessful Inoculation against Hepatitis B,” Dtsch Med Wochenschr, May 17, 1991, 116(20): 797.
Buddle, BM et al, “Contagious Ecthyma Virus-Vaccination Failures”, Am J Vet Research, Feb 1984, 45(2):263-266.
Mathias, R G, “Whooping Cough In Spite of Immunization”, Can J Pub Health, 1978 Mar/Apr; 69(2):130-132.
Osterholm, MT, et al, “Lack of Efficacy of Haemophilus b Polysacharide Vaccine in Minnesota”, JAMA, 1988 Sept 9; 260(10:1423-1428.
Johnson, RH, et al, “Nosocomial Vaccinia Infection”, West J Med, Oct 1976, 125(4):266-270.
Basa, SN, “Paralytic Poliomyelitis Following Inoculation With Combined DTP Prophylactic. A review of Sixteen cases with Special Reference to Immunization Schedules in Infancy”, J Indian Med Assoc, Feb 1, 1973, 60:97-99.
Pathel, JC, et al, “Tetanus Following Vaccination Against Small-pox”, J Pediatr, Jul 1960; 27:251-263.
Favez, G, “Tuberculous Superinfection Following a Smallpox Re-Vaccination”, Praxis, July 21, 1960; 49:698-699.
Quast, Ute, and Hennessen, “Vaccine-Induced Mumps-like Diseases”, Intern Symp on Immunizations , Development Bio Stand, Vol 43, p 269-272.
Forrest, J M, et al, “Clinical Rubella Eleven months after Vaccination,” Lancet, Aug 26, 1972, 2:399-400.
Dittman, S, “Atypical Measles after Vaccination”, Beitr Hyg Epidemiol, 19891, 25:1-274 (939 ref)
Sen S, et al, “Poliomyelitis in Vaccinated Children”, Indian Pediatr, May 1989, 26(5): 423-429.
Arya, SC, “Putative Failure of Recombinant DNA Hepatitis B Vaccines”, Vaccine, Apr 1989, 7(2): 164-165.
Lawrence, R et al, “The Risk of Zoster after Varicella Vaccination in Children with Leukemia”, NEJM, Mar 3, 1988, 318(9): 543-548.
Na, “DPT Vaccination and Sudden Infant Death – Tennessee, US Dept HEW, MMWR Report, Mar 23, 1979, vol 28(11): 132.
Arevalo, “Vaccinia Necrosum. Report on a Fatal Case”, Bol Ofoc Sanit Panamer, Aug 1967, 63:106-110.
Connolly, J H, Dick, G W, Field, CM, “A Case of Fatal Progressive Vaccinia”, Brit Med Jour, 12 May 1962; 5288:1315-1317.
Aragona, F, “Fatal Acute Adrenal Insufficiency Caused by Bilateral Apoplexy of the Adrenal Glands (WFS) following Anti-poliomyelitis Vaccination”, Minerva Medicolegale, Aug 1960; 80:167-173.
Moblus, G et al, “Pathological-Anatomical Findings in Cases of Death Following Poliomyelitis and DPT Vaccination”, Dtsch Gesundheitsw, Jul 20, 1972, 27:1382-1386.
NA, “Immunizations and Cot Deaths”, Lancet, Sept 25, 1982, np.
Goetzeler, A, “Fatal Encephalitis after Poliomyelitis Vaccination”, 22 Jun 1961, Muenchen Med Wschr, 102:1419-1422.
Fulginiti, V, “Sudden Infant Death Syndrome, Diphtheria-Tetanus Toxoid-Pertussis Vaccination and Visits to the Doctor: Chance Association or Cause and Effect?”, Pediatr Infect Disorder, Jan-Feb 1983, 2(1): 7-11.
Baraff, LJ, et al, “Possible Temporal Association Between Diphtheria-tetanus toxoid-Pertussis Vaccination and Sudden Infant Death Syndrome”, Pediatr Infect Disorder, Jan-Feb 1983, 2(1): 5-6.
Reynolds, E, “Fatal Outcome of a Case of Eczema Vaccinatum”, Lancet, 24 Sept 1960, 2:684-686.
Apostolov. et al, “Death of an Infant in Hyperthermia After Vaccination”, J Clin Path, Mar 1961, 14:196-197.
Bouvier-Colle, MH, “Sex-Specific Differences in Mortality After High-Titre Measles Vaccination”, Rev Epidemiol Sante Publique, 1995; 43(1): 97.
Stewart GT, “Deaths of infants after triple vaccine.”, Lancet 1979 Aug 18;2(8138):354-355.
Flahault A, “Sudden infant death syndrome and diphtheria/tetanus toxoid/pertussis/poliomyelitis immunisation.”, Lancet 1988 Mar 12;1(8585):582-583.
Larbre, F et al, “Fatal Acute Myocarditis After Smallpox Vaccination”, Pediatrie, Apr-May 1966, 21:345-350.
Mortimer EA Jr, “DTP and SIDS: when data differ”, Am J Public Health 1987 Aug; 77(8):925-926.
Deutsch J, ” [Temperature changes after triple-immunization in infant age],” Padiatr Grenzgeb 1976;15(1):3-6. [Article in German]
NA, “[Temperature changes after triple immunization in childhood],” Padiatr Grenzgeb 1976;15(1):7-10. [Article in German]
Burmistrova AL, “[Change in the non-specific resistance of the body to influenza and acute respiratory diseases following immunization diphtheria-tetanus vaccine],” Zh Mikrobiol Epidemiol Immunobiol 1976; (3):89-91. [Article in Russian]
Kaga, “Unilateral Total Loss of Auditory and Vestibular Function as a Complication of Mumps Vaccination”, Int J Ped Oto, Feb 1998, 43(1):73-73
Nabe-Nielsen, Walter, “Unilateral Total Deafness as a Complication of the Measles- Mumps- Rubella Vaccination”, Scan Audio Suppl, 1988, 30:69-70
Hulbert, et al, “Bilateral Hearing Loss after Measles and Rubella Vaccination in an Adult”, NEJM, 1991 July, 11;325(2):134
Healy, “Mumps Vaccine and Nerve Deafness”, Am J Disorder Child, 1972 Jun; 123(6):612
Jayarajan, Sedler, “Hearing Loss Following Measles Vaccination”, J Infect, 1995 Mar; 30(2):184-185
Pialoux, P et al, “Vaccinations and Deafness”, Ann Otolaryng (Paris), Dec 1963, 80:1012-1013.
Angerstein, W, et al, “Solitary Hearing and Equilibrium Damage After Vaccinations”, Gesundheitswesen, May 1995, 57(5): 264-268.
Brodsky, Stanievich, “Sensorineural Hearing Loss Following Live Measles Virus Vaccination”, Int J Ped Oto, 1985 Nov; 10(2):159-163
Koga, et al, “Bilateral Acute Profound Deafness After MMR Vaccination- Report of a Case”, Nippon Jibiin Gakkai Kai, 1991 Aug;94(8):1142-5
Seiferth, LB, “Deafness after Oral Poliomyelitis Vaccination – a Case Report and Review”, HNO, 1977 Aug; 25(8): 297-300
Pantazopoulos, PE, “Perceptive Deafness Following Prophylactic use of Tetanus anittoxin”, Laryngoscope, Dec 1965, 75:1832-1836.
Zimmerman, W, “Observation of a case of Acute Bilateral Hearing Impairment Following Preventive Poliomyelitis Vaccination (type 3)”, Arch Ohr Nas Kehlkopfheilk, 1965, 185:723-725.
Jacquot, C et al, “Renal Risk in Vaccination”, Nouv Presse Med, Nov 6, 1982, 11(44):3237-3238.
Giudicelli, et al, “Renal Risk in Vaccination”, Presse Med, Jun 11, 1982, 12(25):1587-1590.
Tan, SY, et al, “Vaccine Related Glomerulonephritis”, BMJ, Jan 23, 1993, 306(6872):248.
Pillai, JJ, et al, “Renal Involvement in Association with Post-vaccination Varicella”, Clin Infect Disorder, Dec 1993, 17(6): 1079-1080.
Eisinger, AJ et al, “Acute Renal Failure after TAB and Cholera Vaccination”, B Med J, Feb 10, 1979, 1(6160):381-382.
Silina, ZM, et al, “Causes of Postvaccinal Complications in the Kidneys in Young Infants”, Pediatria, Dec 1978, (12):59-61.
Na, “Albuminurias”, Concours Med, Mar 1964, 85:5095-5098.
Oyrl, A, et al, “Can Vaccinations Harm the Kidney?”, Clin Nephrol, 1975, 3(5):204-205.
Mel’man Nia, “[Renal lesions after use of vaccines and sera].” Vrach Delo 1978 Oct;(10):67-9, [Article in Russian]
Silina ZM, Galaktionova TIa, Shabunina NR, “[Causes of postvaccinal complications in the kidneys in young infants].” Pediatriia 1978 Dec;(12):59-61, [Article in Russian]
Silina EM, et al, “[Some diseases of the kidneys in children during the 1st year of life, following primary smallpox vaccination and administration of pertusis-diphtheria-tetanus vaccine].” Vopr Okhr Materin Det 1968 Mar; 13(3):79-80, [Article in Russian]
Illingsworth R, Skin rashes after triple vaccine,” Arch Dis Child 1987 Sep; 62(9):979.
Lupton GP, “Discoid lupus erythematosus occurring in a smallpox vaccination scar,” J Am Acad Dermatol, 1987 Oct; 17(4):688-690.
Kompier, A J, “Some Skin Diseases caused by Vaccinia Virus [Smallpox],” Ned Milt Geneesk T, 15:149-157, May 1962.
Weber, G et al, “Skin Lesions Following Vaccinations,” Deutsch Med Wschr, 88:1878-1886, S7 Sept 1963.
Copeman, P W, “Skin Complications of Smallpox Vaccination,” Practitioner, 197:793-800, Dec 1966.
Denning, DW, et al, “Skin Rashes After Triple Vaccine,” Arch Disorder Child, May 1987, 62(5): 510-511.
Sterler, HC, et al, “Outbreaks of Group A Steptococcal Abcesses Following DTP Vaccination”, Pediatrics, Feb 1985, 75(2):299-303.
DiPiramo, D, et al, “Abcess Formation at the Site of Inoculation of Calmette-Guerin Bacillus (BCG),” Riv Med Aeronaut Spaz, Jul-Dec 1981, 46(3-4):190-199.
Caileba, A et al, “Shock associated with Disseminated Intravascular Coagulation Syndrome following Injection of DT.TAB Vaccine, Prese Med, Sept 15, 1984, 13(3):1900. Vaccines:
Pathel, JC, et al, “Tetanus Following Vaccination Against Small-pox”, J Pediatr, Jul 1960; 27:251-263.
Favez, G, “Tuberculous Superinfection Following a Smallpox Re-Vaccination”, Praxis, July 21, 1960; 49:698-699.
Bonifacio, A et al, “Traffic Accidents as an expression of “Iatrogenic damage”, Minerva Med, Feb 24, 1971, 62:735-740.
Baker, J et al, “Accidental Vaccinia: Primary Inoculation of a Scrotum”, Clin Pediatr (Phila), Apr 1972, 11:244-245.
Edwards, K, “Danger of Sunburn Following Vaccination”, Papua New Guinea Med J, Dec 1977, 20(4):203.
Stroder, J, “Incorrect Therapy in Children”, Folia Clin Int (Barc), Feb 1966, 16:82-90.
Wehrle PF, “Injury associated with the use of vaccines,” Clin Ther 1985;7(3):282-284.
Alberts ME, “When and where will it stop”, Iowa Med 1986 Sep; 76(9):424.
Breiman RF, Zanca JA, “Of floors and ceilings — defining, assuring, and communicating vaccine safety”, Am J Public Health 1997 Dec;87(12):1919-1920.
Stewart, AM, et al, “Aetiology of Childhood Leukaemia”, Lancet, 16 Oct, 1965, 2:789-790.
Nelson, ST, “John Hutchinson On Vaccination Syphilis (Hutchinson, J)”, Arch Derm, (Chic), May 1969, 99:529-535.
Mather, C, “Cotton Mather Anguishes Over the Consequences of His Son’s Inoculation Against Smallpox”, Pediatrics, May 1974; 53:756.
Thoman M, “The Toxic Shot Syndrome”, Vet Hum Toxicol, Apr 1986, 28(2):163-166.
Johnson, RH, et al, “Nosocomial Vaccinia Infection”, West J Med, Oct 1976, 125(4):266-270.
Heed, JR, “Human Immunization With Rabies Vaccine in Suckling Mice Brain,” Salud Publica, May-Jun 1974, 16(3): 469-480.
Tesovic, G et al, “Aseptic Meningitis after Measles, Mumps and Rubella Vaccine”, Lancet, Jun 12, 1993, 341(8859):1541.
Buddle, BM et al, “Contagious Ecthyma Virus-Vaccination Failures”, Am J Vet Research, Feb 1984, 45(2):263-266.
Freter, R et al, “Oral Immunization And Production of Coproantibody in Human Volunteers”, J Immunol, Dec 1963, 91:724-729.
NA, “Vaccination, For and Against”, 1964, Belg T Geneesk, 20:125-130.
Sahadevan, MG et al, “Post-vaccinal Myelitis”, J Indian Med Ass, Feb 16, 1966, 46:205-206.
Castan, P et al, “Coma Revealing an acute Leukosis in a child, 15 days after an Oral Anti-poliomyelitis Vaccination,” Acta Neurol Bekg, May 1965, 65:349-367.
Stickl, H, et al, “Purulent [pus] meningitides Following Smallpox Vaccination. On the Problem of Post- Vaccinal Decrease of Resistance”, Deutsch Med Wschr, Jul 22, 1966, 91:1307-1310.
CDC. (2016, August 3). Making the vaccine decision. Retrieved February 4, 2017, from Centers for Disease Control and Prevention, https://www.cdc.gov/vaccines/parents/vaccine-decision/index.html
Geier, D. A., Hooker, B. S., Kern, J. K., King, P. G., Sykes, L. K., Geier, M. R., … Spring, S. (2013). A two-phase study evaluating the relationship between Thimerosal-containing vaccine administration and the risk for an autism spectrum disorder diagnosis in the United States. Translational Neurodegeneration, 2(1), 25. doi:10.1186/2047-9158-2-25
Grand View Research. (2017). Vaccine market size projected to reach $77.5 Billion by 2024. Retrieved February 2, 2017, from https://www.grandviewresearch.com/press-release/global-vaccine-market
Hooker, B. S. (2014). Measles-mumps-rubella vaccination timing and autism among young african american boys: A reanalysis of CDC data. Translational Neurodegeneration, 3(1), 16. doi:10.1186/2047-9158-3-16
Kern, J., Geier, D., Audhya, T., King, P., Sykes, L., & Geier (2012). Evidence of parallels between mercury intoxication and the brain pathology in autism. Acta neurobiologiae experimentalis., 72(2), 113–53. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22810216
Bichel, “Post-vaccinial Lymphadenitis Developing into Hodgkin’s Disease”, Acta Med Scand, 1976, Vol 199, p523-525.
Stewart, AM, et al, “Aetiology of Childhood Leukaemia”, Lancet, 16 Oct, 1965, 2:789-790. [Listed under Vaccine Adverse Reactions.]
Glathe, H et al, “Evidence of Tumorigenic Activity of Candidate Cell Substrate in Vaccine Production by the Use of Anti-Lymphocyte Serum”, Development Biol Std, 1977, 34:145-148.
Bolognesi, DP, “Potential Leukemia Virus Subunit Vaccines: Discussion”, Can Research, Feb 1976, 36(2 pt 2):655-656.
Colon, VF, et al, “Vaccinia Necrosum as a Clue to Lymphatic Lymphoma”, Geriatrics, Dec 1968, 23:81-82.
Park-Dincsoy, H et al, “Lymphoid Depletion in a case of Vaccinia Gangrenosa”, Laval Med, Jan 1968, 39:24-26.
Hugoson, G et al, “The Occurrence of Bovine Leukosis Following the Introduction of Babesiosis Vaccination”, Bibl Haemat, 1968, 30:157-161.
Hartstock, , “”Post-vaccinial Lymphadenitis: Hyperplasia of Lymphoid Tissue That Simulates Malignant Lymphomas”, Apr 1968, Cancer, 21(4):632-649.
Allerberger, F, “An Outbreak of Suppurative Lymphadenitis Connected with BCG Vaccination in Austria- 1990/1991,” Am Rev Respir Disorder, Aug 1991, 144(2) 469.
Omokoku B, Castells S, “Post-DPT inoculation cervical lymphadenitis in children.” N Y State J Med 1981 Oct;81(11):1667-1668.
Knuutila, S et al, “An Increased Frequency of Chromosomal Changes and SCE’s in Cultured Lymphocytes of 12 Subjects Vaccinated Against Smallpox,” Hum Genet, 1978 Feb 23; 41(1):89-96.
Cherkeziia, SE, et al, “Disorders in the Murine Chromosome Apparatus Induced By Immunization with a Complex of Anti-viral Vaccines,” Vopr Virusol, 1979 Sept Oct, (5):547-550.
Romanov, V A, et al, "Role of Auto-immune Processes in the Pathogenesis of Post-Vaccinal Lesions of the Nervous System", Oct 1977, Zh Mikrobiol Epidemiol Immunobiol, 10:80-83.
Grachev, V P, et al, "Formation of Auto-antibodies in Laboratory Animals After Inoculation of Viruses With Different Virulence. I. Results of Studies ..., July 1973, Acta Virol (Praha), 17:319-326.
Movsesiants, AA, et al, "Experimental Study of the Ability of Different Strains of Vaccinia Virus to Induce Auto-Antibody Formation", Vopr Virusol, May-Jun 1975; (3):297-302.
Sinaniotis, et al, "Diabetes Mellitus after Mumps Vaccination", Arc Dis Child, 1975, 50:749.66
Polster, H, "Diabetes insipidus after Smallpox vaccination", Z Aerztl Fortbild (Jena), 1 Apr 1966, 60:429-432.
Patan, "Postvaccinal Severe Diabetes Mellitus", Ter Arkh, Jul 1968, 40:117-118.
Classen, JB, MD, "The Timing of Immunization Affects The Development of Diabetes in Rodents", Autoimmunity, 1996, 24:137-145.
Classen JB, "The diabetes epidemic and the hepatitis B vaccines," N Z Med J, 109(1030):366 1996 Sep 27. [letter]
Classen JB, “Childhood immunisation and diabetes mellitus,” N Z Med J, 109(1022):195 1996 May 24 [letter]
Poutasi K, ” Immunisation and diabetes,” N Z Med J 1996 Jul 26;109(1026):283. [letter; comment] Other Articles Linking Diabetes to Vaccines:
Dokheel, T M, “An Epidemic of Childhood Diabetes in the United States? Evidence from ….”, Diabetes Care, 1993, 16:1606-1611.
Parent ME, et al, “Bacille Calmette-Guerin vaccination and incidence of IDDM in Montreal, Canada,” Diabetes Care 1997 May; 20(5):767-772.
House DV, Winter WE, “Autoimmune diabetes. The role of auto-antibody markers in the prediction and prevention of insulin-dependent diabetes mellitus,” Clin Lab Med 1997 Sep; 17(3):499-545.
Zeigler, M et al , “[Autoantibodies in type 1 diabetes mellitus]” Z Arztl Fortbild (Jena). 1994 Aug; 88(7-8):561-5
Bondarev, VN et al, “The Changes of the Nervous System in Children After Vaccination”, Pediatria, Jun 1969; 48:20-24.
Ehrengut W, “Central nervous sequelae of vaccinations,” Lancet 1986 May 31;1(8492):1275-1276.
Provvidenza, G et al, [On a Case of Benign Acute Cerebellar Ataxia in Childhood], Arch Ital Sci Med Trop, 43:189-194, Apr 1962.
Katsilambros, L, “[The Phenomenom of Apathy in Man and Animals After the Injection of Viruses in Very High Doses. Clinical Data]“, Rev Med Moyen Orient, 20:539-546, Nov – Dec 1963.
Eggers, C, “Autistic Syndrome (Kanner) And Vaccinations against Smallpox”, Klin Paediatr, Mar 1976, 188(2):172-180.
Kiln MR, “Autism, inflammatory bowel disease, and MMR vaccine.” Lancet 1998 May 2;351(9112):1358.
Selway, “MMR vaccination and autism 1998. Medical practitioners need to give more than reassurance.” BMJ 1998 Jun 13;316(7147):1824.
Nicoll A, Elliman D, Ross E, “MMR vaccination and autism 1998,” MJ 1998 Mar 7;316(7133):715-716.
Lindley K J, Milla PJ, “Autism, inflammatory bowel disease, and MMR vaccine.”Lancet 1998 Mar 21;351(9106):907-908.
Bedford H, et al, “Autism, inflammatory bowel disease, and MMR vaccine.” Lancet 1998 Mar 21;351(9106):907.
Vijendra K. Singh, Sheren X. Lin, and Victor C. Yang, “Serological Association of Measles Virus and Human Herpesvirus-6 with Brain Autoantibodies in Autism,” Clinical Immunology and Immunopathology, Oct 1998, Vol. 89, No. 1, p 105-108.
Herroelen, L et al, "Central-Nervous-System Demyelination After Immunization with Recombinant Hepatitis B Vaccine", Lancet, Nov 9, 1991, 338(8776):1174-1175.
Kaplanski G, Retornaz F, Durand J, Soubeyrand J, "Central nervous system demyelination after vaccination against hepatitis B and HLA haplotype." J Neurol Neurosurg Psychiatry 1995 Jun; 58(6):758-759.
Matyszak MK, Perry VH, "Demyelination in the central nervous system following a delayed-type hypersensitivity response to bacillus Calmette-Guerin." Neuroscience 1995 Feb;64(4):967-977.
Tornatore CS, Richert JR, "CNS demyelination associated with diploid cell rabies vaccine." Lancet 1990 Jun 2;335(8701):1346-1347.
Adams, JM et al, "Neuromyelitis Optica: Severe Demyelination Occurring Years After Primary Smallpox Vaccinations", Rev Roum Neurol, 1973, 10:227-231.
Hirtz DG, Nelson KB, Ellenberg J H, "Seizures following childhood immunizations", Pediatr 1983 Jan; 102(1):14-18.
Cherry JD, Holtzman AE, Shields WD, Buch D, Nielsen, "Pertussis immunization and characteristics related to first seizures in infants and children,"J Pediatr 1993 Jun;122(6):900-903.
Coplan J, "Seizures following immunizations," J Pediatr 1983 Sep;103(3):496.
Barkin RM, Jabhour JT, Samuelson J S, "Immunizations, seizures, and subsequent evaluation," JAMA 1987 Jul 10;258(2):201.
Griffin MR, et al, "Risk of seizures after measles-mumps-rubella immunization," Pediatrics 1991 Nov;88(5):881-885.
Griffin MR, et al, "Risk of seizures and encephalopathy after immunization with the diphtheria-tetanus-pertussis vaccine," JAMA 1990 Mar 23-30;263(12):1641-1645.
Cizewska S, Huber Z, Sluzewski W, "[Prophylactic inoculations and seizure activity in the EEG],” Neurol Neurochir Pol 1981 Sep-Dec;15(5-6):553-557. [Article in Polish]
Huttenlocher PR, Hapke RJ, “A follow-up study of intractable seizures in childhood.” Ann Neurol 1990 Nov; 28(5):699-705.
Blumberg DA, “Severe reactions associated with diphtheria-tetanus-pertussis vaccine: detailed study of children with seizures, hypotonic-hypo-responsive episodes, high fevers, and persistent crying.”Pediatrics 1993 Jun; 91(6):1158-1165.
Prensky AL, et al, “History of convulsions and use of pertussis vaccine,” J Pediatr 1985 Aug; 107(2):244-255.
Baraff LJ, “Infants and children with convulsions and hypotonic-hypo-responsive episodes following diphtheria-tetanus-pertussis immunization: follow-up evaluation,” Pediatrics 1988 Jun; 81(6):789-794.
Jacobson V, “Relationship of pertussis immunization to the onset of epilepsy, febrile convulsions and central nervous system infections: a retrospective epidemiologic study,” Tokai J Exp Clin Med 1988;13 Suppl: 137-142.
Cupic V,et al, “[Role of DTP vaccine in the convulsive syndromes in children],” Lijec Vjesn 1978 Jun; 100(6):345-348. [Article in Serbo-Croatian (Roman)]
Pokrovskaia NIa, “[Convulsive syndrome in DPT vaccination (a clinico-experimental study)],” Pediatriia 1983 May;(5):37-39. [Article in Russian]
Ballerini, Ricci, B, et al, “On Neurological Complications of Vaccination, With Special Reference to Epileptic Syndromes,” Riv Neurol, Jul-Aug 1973, 43:254-258.
Wolf SM, Forsythe A, “Epilepsy and mental retardation following febrile seizures in childhood,” Acta Paediatr Scand 1989 Mar;78(2):291-295.
Iwasa, S et al, “Swelling of the Brain in Mice Caused by Pertussis … Quantitative Determination and the Responsibility of the Vaccine”, Jpn J Med Sci Biol, 1985 , 38(2):53-65.
Mathur R, Kumari S, “Bulging fontanel following triple vaccine.” Indian Pediatr 1981 Jun;18(6):417-418.
Barry W, Lenney W, Hatcher G, “Bulging fontanelles in infants without meningitis.” Arch Dis Child 1989 Apr;64(4):635-636.
Shendurnikar N, “Bulging fontanel following DPT” Indian Pediatr 1986 Nov;23(11):960.
Gross TP, Milstien JB, Kuritsky JN, “Bulging fontanelle after immunization with diphtheria-tetanus-pertussis vaccine and diphtheria-tetanus vaccine.” J Pediatr 1989 Mar;114(3):423-425.
Jacob J, Mannino F, “Increased intracranial pressure after diphtheria, tetanus, and pertussis immunization.” Am J Dis Child 1979 Feb;133(2):217-218.
Dugmore, WN, “Bilateral Oedema at the Posterior Pole. Hypersensitivity Reaction to Alavac P injection.” Br J Ophthalmol, Dec 1972, 55:848-849.
Nedar P R, and Warren, R J, “Reported Neurological Disorders Following Live Measles Vaccine”, 1968, Ped, 41:997-1001.
Paradiso, G et al, “Multifocal Demyelinating Neuropathy after Tetanus Vaccine”, Medicina (B Aires), 1990, 50(1):52-54.
Landrigan, PJ, Whitte, J, “Neurologic Disorders Following Live Measles-virus Vaccination”, JAMA, Mar 26, 1973, v223(13):1459-1462.
Turnbull, H M, “Encephalomyelitis Following Vaccination”, Brit Jour Exper Path, 7:181, 1926.
Kulenkampff, M et al, “Neurological Complications of Pertussis Inoculation”, Arch Dis Child, 1974, 49:46.
Strom, J, “Further Experience of Reactions, Especially of a Cerebral Nature in Conjunction with Triple Vaccination”, Brit Med Jour, 1967, 4:320-323.
Berg, J M, “Neurological Complications of Pertussis Immunization,” Brit Med Jour, July 5,1958; p 24.
Bondarev, VN et al, “The Changes of the Nervous System in Children After Vaccination”, Pediatria, Jun 1969; 48:20-24.
Badalian, LO, “Vaccinal Lesions of the Nervous System in Children,” Vop Okhr Materin Dets, Dec 1959, 13:54-59
Lorentz, IT, et al, “Post-Vaccinal Sensory Polyneuropathy with Myoclonus”, Proc Aust Ass Neurol, 1969, 6:81-86.
Trump, R C, White, T R, “Cerebellar Ataxia Presumed Due To Live Attenuated Measles Virus Vaccine,” JAMA, 1967, 199:165-166.
Allerdist, H, “Neurological Complications Following Measles Vaccination”, Inter Symp, Brussels, 1978, Development Biol Std, Vol 43, 259-264.
Finley, K H, “Pathogenesis of Encephalitis Occurring With Vaccination, Variola and Measles, Arch Neur and Psychologist, 1938; 39:1047-1054.
Froissart, M et al, “Acute Meningoencephalitis Immediately after an Influenza Vaccination”, Lille Med, Oct 1978, 23(8):548-551.
Pokrovskaia, Nia, et al, “Neurological Complications in Children From Smallpox Vaccination”, Pediatriia, Dec 1978, (12):45-49.
Allerdist, H, “Neurological Complications Following Measles Virus Vaccination. Evaluation of the Cases seen Between 1971-1977″, Monatsschr Kinderheilkd, Jan 1979, 127(1): 23-28.
Ehrengut, W et al, “On Convulsive Reactions Following Oral vaccination Against Polio”, Klin Paediatr, May 1979, 191(3):261-270.
Naumova, R P, et al, “Encephalitis Developing After Vaccination without a Local Skin Reaction”, Vrach Delo, Jul 1979, (7):114-115.
Goswamy, BM, “Neurological Complications After Smallpox Vaccination”, J Ass Phys India, Jan 1969, 17:41-43.
Schchelkunov, SN et al, “The Role of Viruses in the Induction of Allergic Encephalomyelitis,” Dokl Akad Nauk SSSR, 1990,315(1):252-255.
Walker AM, “Neurologic events following diphtheria-tetanus-pertussis immunization,” Pediatrics 1988 Mar;81(3):345-349.
Shields WD, et al, “Relationship of pertussis immunization to the onset of neurologic disorders: a retrospective epidemiologic study,” J Pediatr 1988 Nov; 113(5):801-805.
Wilson J, “Proceedings: Neurological complications of DPT inoculation in infancy,” Arch Dis Child 1973 Oct; 48(10):829-830.
Iakunin IuA, “[Nervous system complications in children after preventive vaccinations],” Pediatriia 1968 Nov; 47(11):19-26. [Article in Russian]
Greco D, et al, “Case-control study on encephalopathy associated with diphtheria-tetanus immunization in Campania, Italy,” Bull World Health Organ 1985;63(5):919-925.
Ehrengut W, “Bias in evaluating CNS complications following pertussis immunization.” Acta Paediatr Jpn, 1991 Aug; 33(4):421-427.
Hiner, E E, Frasch, C E, “Spectrum of Disease Due to Haemophilus Influenza Type B Occurring in Vaccinated Children”, J Infect Disorder, 1988 Aug; 158(2): 343-348.
Olin P, Romanus, V, Storsaeter, J, “Invasive Bacterial Infections During an Efficiacy Trial of Acellular Pertussis Vaccines — Implications For Future Surveilance In Pertussis Vaccine Programmes”, Tokai J Exp Clin Med, 1988; 13 Suppl: 143-144.
Storsaeter, J, et al, “Mortality and Morbidity From Invasive Bacterial Infections During a Clinical Trial of Acellular Pertussis Vaccines in Sweden”, Pediatr Infect Disorder J, 1988 Sept; 7(9):637-645.
Vadheim, CM, et al, “Effectiveness and Safety of an Haemophilus Influenzae type b Conjugate Vaccine (PRP-T) in Young Infants. Kaiser-UCLA Vaccine Study Group,” Pediartics, 1993 Aug; 92(2):272-279.
Stickl, H, “Estimation of Vaccination Damage”, Med Welt, Oct 14, 1972, 23:1495-1497.
Waters, VV, et al, “Risk Factors for Measles in a Vaccinated Population”, JAMA, Mar 27, 1991, 265(12): 1527.
Stickl, H, “Iatrogenic Immuno-suppression as a Result of Vaccination”, Fortschr Med, Mar 5, 1981, 99(9);289-292.
Nkowane, et al, “Vaccine-Associated Paralytic Poliomyelitis, US 1973 through 1984, JAMA, 1987, Vol 257:1335-1340.
Quast, et al, “Vaccine Induced Mumps-like Diseases”, nd, Int Symp on Immun, Development Bio Stand, Vol 43, p269-272.
Green, C et al, “A Case of Hepatitis Related to Etretinate Therapy and Hepatitis B Vaccine”, Dermatologica, 1991, 182(2):119-120.
Shasby, DM, et al, “Epidemic Measles in Highly Vaccinated Population”, NEJM, Mar 1977, 296(11): 585-589.
Tesovic, G et al, “Aseptic Meningitis after Measles, Mumps and Rubella Vaccine”, Lancet, Jun 12, 1993, 341(8859):1541.
Johnson, RH, et al, “Nosocomial Vaccinia Infection”, West J Med, Oct 1976, 125(4):266-270.
Malengreau, M, “Reappearance of Post-Vaccination Infection of Measles, Rubella, and Mumps. Should Adolescents be re-vaccinated?” Pedaitric, 1992;47(9):597-601 (25 ref)
Basa, SN, “Paralytic Poliomyelitis Following Inoculation With Combined DTP Prophylactic. A review of Sixteen cases with Special Reference to Immunization Schedules in Infancy”, J Indian Med Assoc, Feb 1, 1973, 60:97-99.
Landrigan, PJ et al, “Measles in Previously Vaccinated Children in Illinois”, Ill Med J, Arp 1974, 141:367-372.
NA, “Vaccine-Associated Poliomyelitis”, Med J Aust, Oct 1973, 2:795-796.
Hardy, GE, Jr, et al, “The Failure of a School Immunization Campaign to Terminate an Urban Epidemic of Measles,” Amer J Epidem, Mar 1970; 91:286-293.
Cherry, JD, et al, “A Clinical and Serologic Study of 103 Children With Measles Vaccine Failure”, J Pediatr, May 1973; 82:801-808.
Jilg, W, et al, “Inoculation Failure Following Hepatitis B Vaccination”, Dtsch Med wochenschr, 1990 Oct 12; 115(41):1514-1548.
Plotkin, SA, “Failures of Protection by Measles Vaccine,” J Pediatr, May 1973; 82:798-801.
Bolotovskii, V, et al, “Measles Incidence Among Children Properly Vaccinated Against This Infection”, ZH Mikrobiol Epidemiol Immunobiol, 1974; 00(5):32-35.
Landrigan, PJ, et al, “Measles in Previously Vaccinated Children in Illinois”, Ill Med J, Apr 1974; 141:367-372.
Strebel, P et al, “An Outbreak of Whooping Cough in a Highly Vaccinated Urban Community”, J Trop Pediatr, Mar 1991, 37(2): 71-76.
Forrest, JM, et al, “Failure of Rubella Vaccination to Prevent Congenital Rubella,”Med J Aust, 1977 Jan 15; 1(3): 77.
Jilg, W, “Unsuccessful Vaccination against Hepatitis B”, Dtsch Med Wochenschr, Nov 16, 1990, 115(46):1773.
Coles, FB, et al, “An Outbreak of Influenza A (H3N2) in a Well-Immunized Nursing home Population,” J Am ger Sociologist, Jun 1992, 40(6):589-592.
Jilg, W, et al, “Inoculation Failure following Hepatitis B Vaccination,” Dtsch Med Wochenschr, Oct 12, 1990, 115(41):1545-1548.
Hartmann, G et al, “Unsuccessful Inoculation against Hepatitis B,” Dtsch Med Wochenschr, May 17, 1991, 116(20): 797.
Buddle, BM et al, “Contagious Ecthyma Virus-Vaccination Failures”, Am J Vet Research, Feb 1984, 45(2):263-266.
Mathias, R G, “Whooping Cough In Spite of Immunization”, Can J Pub Health, 1978 Mar/Apr; 69(2):130-132.
Osterholm, MT, et al, “Lack of Efficacy of Haemophilus b Polysacharide Vaccine in Minnesota”, JAMA, 1988 Sept 9; 260(10:1423-1428.
Johnson, RH, et al, “Nosocomial Vaccinia Infection”, West J Med, Oct 1976, 125(4):266-270.
Basa, SN, “Paralytic Poliomyelitis Following Inoculation With Combined DTP Prophylactic. A review of Sixteen cases with Special Reference to Immunization Schedules in Infancy”, J Indian Med Assoc, Feb 1, 1973, 60:97-99.
Pathel, JC, et al, “Tetanus Following Vaccination Against Small-pox”, J Pediatr, Jul 1960; 27:251-263.
Favez, G, “Tuberculous Superinfection Following a Smallpox Re-Vaccination”, Praxis, July 21, 1960; 49:698-699.
Quast, Ute, and Hennessen, “Vaccine-Induced Mumps-like Diseases”, Intern Symp on Immunizations , Development Bio Stand, Vol 43, p 269-272.
Forrest, J M, et al, “Clinical Rubella Eleven months after Vaccination,” Lancet, Aug 26, 1972, 2:399-400.
Dittman, S, “Atypical Measles after Vaccination”, Beitr Hyg Epidemiol, 19891, 25:1-274 (939 ref)
Sen S, et al, “Poliomyelitis in Vaccinated Children”, Indian Pediatr, May 1989, 26(5): 423-429.
Arya, SC, “Putative Failure of Recombinant DNA Hepatitis B Vaccines”, Vaccine, Apr 1989, 7(2): 164-165.
Lawrence, R et al, “The Risk of Zoster after Varicella Vaccination in Children with Leukemia”, NEJM, Mar 3, 1988, 318(9): 543-548.
Na, “DPT Vaccination and Sudden Infant Death – Tennessee, US Dept HEW, MMWR Report, Mar 23, 1979, vol 28(11): 132.
Arevalo, “Vaccinia Necrosum. Report on a Fatal Case”, Bol Ofoc Sanit Panamer, Aug 1967, 63:106-110.
Connolly, J H, Dick, G W, Field, CM, “A Case of Fatal Progressive Vaccinia”, Brit Med Jour, 12 May 1962; 5288:1315-1317.
Aragona, F, “Fatal Acute Adrenal Insufficiency Caused by Bilateral Apoplexy of the Adrenal Glands (WFS) following Anti-poliomyelitis Vaccination”, Minerva Medicolegale, Aug 1960; 80:167-173.
Moblus, G et al, “Pathological-Anatomical Findings in Cases of Death Following Poliomyelitis and DPT Vaccination”, Dtsch Gesundheitsw, Jul 20, 1972, 27:1382-1386.
NA, “Immunizations and Cot Deaths”, Lancet, Sept 25, 1982, np.
Goetzeler, A, “Fatal Encephalitis after Poliomyelitis Vaccination”, 22 Jun 1961, Muenchen Med Wschr, 102:1419-1422.
Fulginiti, V, “Sudden Infant Death Syndrome, Diphtheria-Tetanus Toxoid-Pertussis Vaccination and Visits to the Doctor: Chance Association or Cause and Effect?”, Pediatr Infect Disorder, Jan-Feb 1983, 2(1): 7-11.
Baraff, LJ, et al, “Possible Temporal Association Between Diphtheria-tetanus toxoid-Pertussis Vaccination and Sudden Infant Death Syndrome”, Pediatr Infect Disorder, Jan-Feb 1983, 2(1): 5-6.
Reynolds, E, “Fatal Outcome of a Case of Eczema Vaccinatum”, Lancet, 24 Sept 1960, 2:684-686.
Apostolov. et al, “Death of an Infant in Hyperthermia After Vaccination”, J Clin Path, Mar 1961, 14:196-197.
Bouvier-Colle, MH, “Sex-Specific Differences in Mortality After High-Titre Measles Vaccination”, Rev Epidemiol Sante Publique, 1995; 43(1): 97.
Stewart GT, “Deaths of infants after triple vaccine.”, Lancet 1979 Aug 18;2(8138):354-355.
Flahault A, “Sudden infant death syndrome and diphtheria/tetanus toxoid/pertussis/poliomyelitis immunisation.”, Lancet 1988 Mar 12;1(8585):582-583.
Larbre, F et al, “Fatal Acute Myocarditis After Smallpox Vaccination”, Pediatrie, Apr-May 1966, 21:345-350.
Mortimer EA Jr, “DTP and SIDS: when data differ”, Am J Public Health 1987 Aug; 77(8):925-926.
Deutsch J, ” [Temperature changes after triple-immunization in infant age],” Padiatr Grenzgeb 1976;15(1):3-6. [Article in German]
NA, “[Temperature changes after triple immunization in childhood],” Padiatr Grenzgeb 1976;15(1):7-10. [Article in German]
Burmistrova AL, “[Change in the non-specific resistance of the body to influenza and acute respiratory diseases following immunization diphtheria-tetanus vaccine],” Zh Mikrobiol Epidemiol Immunobiol 1976; (3):89-91. [Article in Russian]
Kaga, “Unilateral Total Loss of Auditory and Vestibular Function as a Complication of Mumps Vaccination”, Int J Ped Oto, Feb 1998, 43(1):73-73
Nabe-Nielsen, Walter, “Unilateral Total Deafness as a Complication of the Measles- Mumps- Rubella Vaccination”, Scan Audio Suppl, 1988, 30:69-70
Hulbert, et al, “Bilateral Hearing Loss after Measles and Rubella Vaccination in an Adult”, NEJM, 1991 July, 11;325(2):134
Healy, “Mumps Vaccine and Nerve Deafness”, Am J Disorder Child, 1972 Jun; 123(6):612
Jayarajan, Sedler, “Hearing Loss Following Measles Vaccination”, J Infect, 1995 Mar; 30(2):184-185
Pialoux, P et al, “Vaccinations and Deafness”, Ann Otolaryng (Paris), Dec 1963, 80:1012-1013.
Angerstein, W, et al, “Solitary Hearing and Equilibrium Damage After Vaccinations”, Gesundheitswesen, May 1995, 57(5): 264-268.
Brodsky, Stanievich, “Sensorineural Hearing Loss Following Live Measles Virus Vaccination”, Int J Ped Oto, 1985 Nov; 10(2):159-163
Koga, et al, “Bilateral Acute Profound Deafness After MMR Vaccination- Report of a Case”, Nippon Jibiin Gakkai Kai, 1991 Aug;94(8):1142-5
Seiferth, LB, “Deafness after Oral Poliomyelitis Vaccination – a Case Report and Review”, HNO, 1977 Aug; 25(8): 297-300
Pantazopoulos, PE, “Perceptive Deafness Following Prophylactic use of Tetanus anittoxin”, Laryngoscope, Dec 1965, 75:1832-1836.
Zimmerman, W, “Observation of a case of Acute Bilateral Hearing Impairment Following Preventive Poliomyelitis Vaccination (type 3)”, Arch Ohr Nas Kehlkopfheilk, 1965, 185:723-725.
Jacquot, C et al, “Renal Risk in Vaccination”, Nouv Presse Med, Nov 6, 1982, 11(44):3237-3238.
Giudicelli, et al, “Renal Risk in Vaccination”, Presse Med, Jun 11, 1982, 12(25):1587-1590.
Tan, SY, et al, “Vaccine Related Glomerulonephritis”, BMJ, Jan 23, 1993, 306(6872):248.
Pillai, JJ, et al, “Renal Involvement in Association with Post-vaccination Varicella”, Clin Infect Disorder, Dec 1993, 17(6): 1079-1080.
Eisinger, AJ et al, “Acute Renal Failure after TAB and Cholera Vaccination”, B Med J, Feb 10, 1979, 1(6160):381-382.
Silina, ZM, et al, “Causes of Postvaccinal Complications in the Kidneys in Young Infants”, Pediatria, Dec 1978, (12):59-61.
Na, “Albuminurias”, Concours Med, Mar 1964, 85:5095-5098.
Oyrl, A, et al, “Can Vaccinations Harm the Kidney?”, Clin Nephrol, 1975, 3(5):204-205.
Mel’man Nia, “[Renal lesions after use of vaccines and sera].” Vrach Delo 1978 Oct;(10):67-9, [Article in Russian]
Silina ZM, Galaktionova TIa, Shabunina NR, “[Causes of postvaccinal complications in the kidneys in young infants].” Pediatriia 1978 Dec;(12):59-61, [Article in Russian]
Silina EM, et al, “[Some diseases of the kidneys in children during the 1st year of life, following primary smallpox vaccination and administration of pertusis-diphtheria-tetanus vaccine].” Vopr Okhr Materin Det 1968 Mar; 13(3):79-80, [Article in Russian]
Illingsworth R, Skin rashes after triple vaccine,” Arch Dis Child 1987 Sep; 62(9):979.
Lupton GP, “Discoid lupus erythematosus occurring in a smallpox vaccination scar,” J Am Acad Dermatol, 1987 Oct; 17(4):688-690.
Kompier, A J, “Some Skin Diseases caused by Vaccinia Virus [Smallpox],” Ned Milt Geneesk T, 15:149-157, May 1962.
Weber, G et al, “Skin Lesions Following Vaccinations,” Deutsch Med Wschr, 88:1878-1886, S7 Sept 1963.
Copeman, P W, “Skin Complications of Smallpox Vaccination,” Practitioner, 197:793-800, Dec 1966.
Denning, DW, et al, “Skin Rashes After Triple Vaccine,” Arch Disorder Child, May 1987, 62(5): 510-511.
Sterler, HC, et al, “Outbreaks of Group A Steptococcal Abcesses Following DTP Vaccination”, Pediatrics, Feb 1985, 75(2):299-303.
DiPiramo, D, et al, “Abcess Formation at the Site of Inoculation of Calmette-Guerin Bacillus (BCG),” Riv Med Aeronaut Spaz, Jul-Dec 1981, 46(3-4):190-199.
Caileba, A et al, “Shock associated with Disseminated Intravascular Coagulation Syndrome following Injection of DT.TAB Vaccine, Prese Med, Sept 15, 1984, 13(3):1900. Vaccines:
Pathel, JC, et al, “Tetanus Following Vaccination Against Small-pox”, J Pediatr, Jul 1960; 27:251-263.
Favez, G, “Tuberculous Superinfection Following a Smallpox Re-Vaccination”, Praxis, July 21, 1960; 49:698-699.
Bonifacio, A et al, “Traffic Accidents as an expression of “Iatrogenic damage”, Minerva Med, Feb 24, 1971, 62:735-740.
Baker, J et al, “Accidental Vaccinia: Primary Inoculation of a Scrotum”, Clin Pediatr (Phila), Apr 1972, 11:244-245.
Edwards, K, “Danger of Sunburn Following Vaccination”, Papua New Guinea Med J, Dec 1977, 20(4):203.
Stroder, J, “Incorrect Therapy in Children”, Folia Clin Int (Barc), Feb 1966, 16:82-90.
Wehrle PF, “Injury associated with the use of vaccines,” Clin Ther 1985;7(3):282-284.
Alberts ME, “When and where will it stop”, Iowa Med 1986 Sep; 76(9):424.
Breiman RF, Zanca JA, “Of floors and ceilings — defining, assuring, and communicating vaccine safety”, Am J Public Health 1997 Dec;87(12):1919-1920.
Stewart, AM, et al, “Aetiology of Childhood Leukaemia”, Lancet, 16 Oct, 1965, 2:789-790.
Nelson, ST, “John Hutchinson On Vaccination Syphilis (Hutchinson, J)”, Arch Derm, (Chic), May 1969, 99:529-535.
Mather, C, “Cotton Mather Anguishes Over the Consequences of His Son’s Inoculation Against Smallpox”, Pediatrics, May 1974; 53:756.
Thoman M, “The Toxic Shot Syndrome”, Vet Hum Toxicol, Apr 1986, 28(2):163-166.
Johnson, RH, et al, “Nosocomial Vaccinia Infection”, West J Med, Oct 1976, 125(4):266-270.
Heed, JR, “Human Immunization With Rabies Vaccine in Suckling Mice Brain,” Salud Publica, May-Jun 1974, 16(3): 469-480.
Tesovic, G et al, “Aseptic Meningitis after Measles, Mumps and Rubella Vaccine”, Lancet, Jun 12, 1993, 341(8859):1541.
Buddle, BM et al, “Contagious Ecthyma Virus-Vaccination Failures”, Am J Vet Research, Feb 1984, 45(2):263-266.
Freter, R et al, “Oral Immunization And Production of Coproantibody in Human Volunteers”, J Immunol, Dec 1963, 91:724-729.
NA, “Vaccination, For and Against”, 1964, Belg T Geneesk, 20:125-130.
Sahadevan, MG et al, “Post-vaccinal Myelitis”, J Indian Med Ass, Feb 16, 1966, 46:205-206.
Castan, P et al, “Coma Revealing an acute Leukosis in a child, 15 days after an Oral Anti-poliomyelitis Vaccination,” Acta Neurol Bekg, May 1965, 65:349-367.
Stickl, H, et al, “Purulent [pus] meningitides Following Smallpox Vaccination. On the Problem of Post- Vaccinal Decrease of Resistance”, Deutsch Med Wschr, Jul 22, 1966, 91:1307-1310.