|
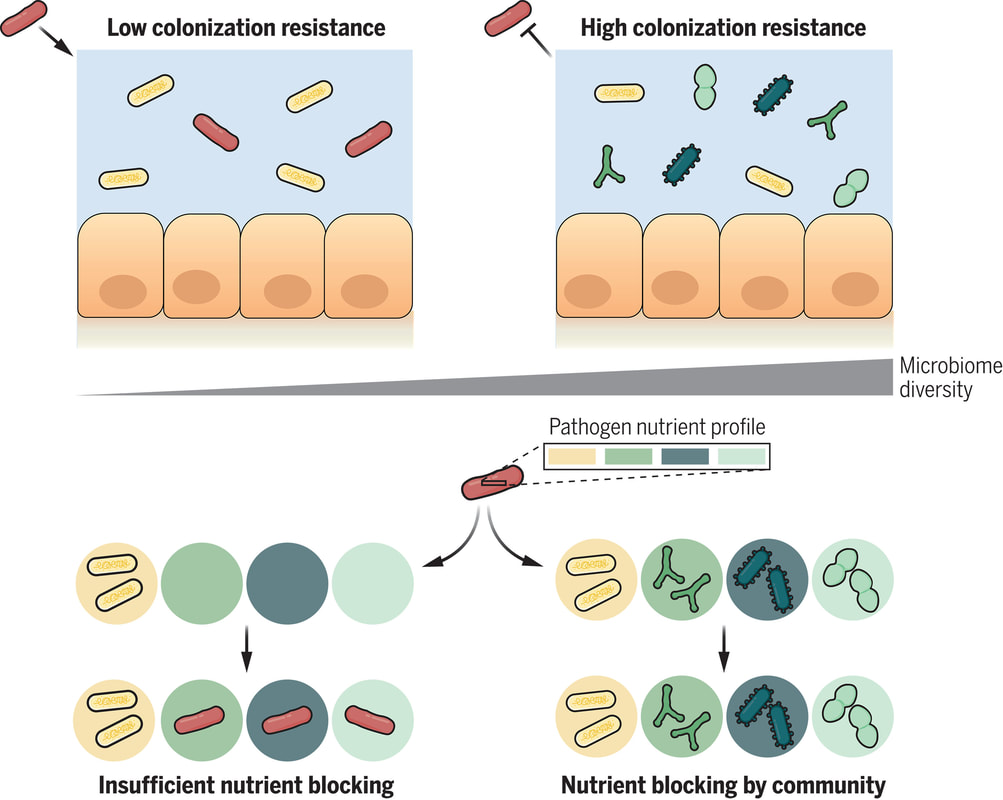
The human gut is teeming with a diverse array of bacteria collectively known as the gut microbiota. Among its many functions, one of the most vital is colonization resistance—the ability to prevent harmful pathogens from taking up residence in the gut and causing disease. However, understanding which microbiota communities are protective and which allow pathogens to thrive has long been a challenge. In a groundbreaking study led by Spragge et al., researchers shed light on the complex dynamics of gut microbiota and their role in colonization resistance against two significant bacterial pathogens: Klebsiella pneumoniae and Salmonella enterica serovar Typhimurium. Their findings, published in Science, unveil the critical importance of microbiome diversity in safeguarding against pathogenic invasion. Traditionally, it was believed that certain individual bacterial species might confer colonization resistance. However, Spragge et al. discovered that the true protective power lies in the collective diversity of the microbiota. They conducted meticulous experiments both in vitro and in gnotobiotic mice (mice that have been raised in a controlled environment where the microbial composition of their gut is precisely known and controlled), evaluating the ability of single bacterial species and increasingly diverse microbiota communities to resist pathogen colonization. Surprisingly, the researchers found that single species alone provided limited protection against the pathogens. It was only when these species were combined into diverse communities consisting of up to 50 different species that colonization resistance was significantly enhanced. This underscores the importance of ecological diversity in promoting gut health. Moreover, the study identified certain key species within these diverse communities that played a pivotal role in bolstering colonization resistance, even though they offered little protection on their own. These key species acted by consuming nutrients required by the pathogens, thereby depriving them of essential resources for growth and establishment in the host. Importantly, Spragge et al. demonstrated that microbiome diversity not only increases the probability of protection against pathogens but also enhances the overlap in nutrient utilization profiles between the microbiota community and the pathogen. This nutrient blocking mechanism serves as a potent defense strategy against pathogenic invasion. The implications of these findings are profound. They provide compelling evidence for the health benefits of a diverse gut microbiome and offer insights into the rational design of pathogen-resistant microbiota communities. By harnessing the protective power of microbiome diversity, we may pave the way for innovative strategies to combat infectious diseases and promote overall gut health. In conclusion, Spragge et al.'s study unveils the intricate interplay between microbiome diversity and colonization resistance, highlighting the collective strength of diverse bacterial communities in defending against pathogenic threats. This research not only expands our understanding of gut microbiota dynamics but also holds promise for the development of novel therapeutics aimed at fortifying the body's natural defenses against infections. referencesSpragge, Frances, et al. “Microbiome Diversity Protects against Pathogens by Nutrient Blocking.” Science, vol. 382, no. 6676, 15 Dec. 2023, https://doi.org/10.1126/science.adj3502.
0 Comments
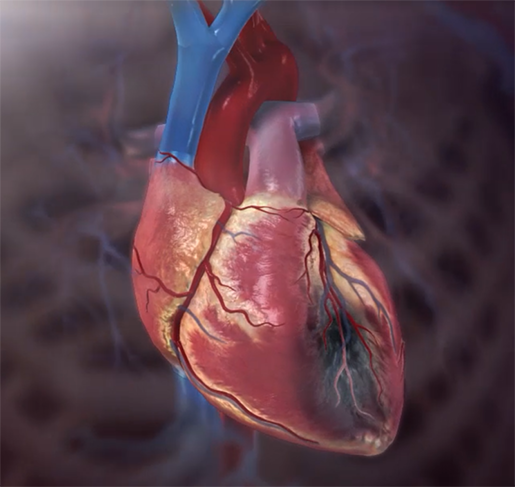
This article challenges the conventional understanding of heart disease, particularly the widely accepted theory that attributes its cause primarily to events occurring in the coronary arteries. Instead, a paradigm shift is proposed, contending that a deeper understanding of heart disease, encompassing angina, unstable angina, and myocardial infarction (heart attack), necessitates a focus on events within the myocardium, the muscular tissue of the heart. Over the past decades, the prevailing belief in the coronary artery theory has led to costly surgical interventions, widespread medication use with questionable benefits, and dietary recommendations that may exacerbate rather than alleviate the problem. By delving into the precise pathophysiological events that underlie heart attacks, we can uncover alternative approaches to prevention and treatment, such as adopting a "Nourishing Traditions"-style diet and utilizing safe and affordable medicines like g-strophanthin. Furthermore, this shift in perspective prompts us to confront broader issues, including the impact of modern lifestyles on human health, the need for a new medical paradigm, and the importance of ecological consciousness. Ultimately, reexamining the root causes of heart disease offers a pathway to addressing this pervasive health challenge and forging a healthier future for all. The information is summarized based on the work of Dr. Thomas Cowan, vice president of the Physicians Association for Anthroposophical Medicine and is a founding board member of the Weston A. Price Foundation. During his career he has studied and written about many subjects in medicine. These include nutrition, homeopathy, anthroposophical medicine, and herbal medicine. Challenging the Conventional model: Revisiting the Causes of Heart AttacksThe traditional understanding of heart attacks, largely centered on arterial blockage due to plaque buildup, has faced challenges in recent years. Initially, it was believed that blockages in the major coronary arteries led to oxygen deficiency in the heart, causing chest pain (angina) and eventually progressing to a heart attack. This simplistic view prompted invasive procedures like angioplasty, stents, and coronary bypass surgery as standard treatments. However, clinical observations and research findings have cast doubts on this approach. Anecdotal evidence (admittedly low quality evidence) from a trial in rural Alabama revealed surprising outcomes among individuals with single artery blockages. Contrary to expectations, less than 10% of those who experienced heart attacks did so in the region of the heart supplied by the blocked artery. Similarly, a comprehensive study conducted by the Mayo Clinic highlighted the limited efficacy of bypass surgery in preventing future heart attacks. While the procedure offered relief from chest pain, it did not significantly reduce the risk of subsequent heart events, except in high-risk patients. Contrary to popular belief, blockages exceeding 90% are often compensated for by collateral blood vessels, which develop over time to ensure uninterrupted blood flow to the heart. This extensive network of collateral vessels serves as a natural bypass system, mitigating the impact of arterial blockages on blood circulation. However, diagnostic procedures like coronary angiograms, which rely on injecting heavy dye into the arteries, often fail to accurately assess the extent of blockages and the true blood flow in the heart. As a result, many patients undergo invasive treatments such as bypass surgery, stents, or angioplasty based on misleading information about the severity of their arterial blockages. Moreover, studies have shown that these procedures provide minimal benefit, if any, to patients, particularly those with minimally symptomatic blockages exceeding 90%. Despite the widespread use of these interventions, their efficacy in restoring blood flow and preventing heart attacks remains questionable. These revelations underscore the need for a reevaluation of conventional treatment strategies and a deeper exploration of the underlying mechanisms behind heart attacks. Rather than focusing solely on arterial blockages, a more holistic approach that considers factors beyond plaque buildup may offer greater insights into the prevention and management of heart disease. Beyond the Coronary Artery TheoryThe prevailing focus in cardiology has long been on the stable, progressing plaque within the coronary arteries, deemed responsible for heart attacks. However, recent insights challenge this notion, redirecting attention to the unpredictable nature of unstable plaques. Unlike their calcified counterparts, unstable plaques are soft and prone to rapid evolution, abruptly occluding arteries and triggering downstream oxygen deficits, angina, and ischemia. These vulnerable plaques are believed to be a blend of inflammatory buildup and low-density lipoprotein (LDL), the primary targets of statin drugs. Consequently, the widespread adoption of statin therapy is advocated as a preventive measure against heart attacks, fueled by angiogram studies purportedly showcasing the prevalence of unstable plaques as the leading cause of myocardial infarctions (MIs). Yet, autopsies and pathology studies present a different narrative. Thrombosis, deemed crucial in precipitating MIs, is found in only a fraction of cases upon meticulous examination. Furthermore, measurements of myocardial oxygen levels during MIs reveal no discernible deficit, challenging the conventional understanding of ischemia as the primary mechanism. While thrombosis does occur in conjunction with MIs, its occurrence in less than half of cases underscores the inadequacy of attributing MIs solely to arterial blockages. The timing of thrombosis, often post-MI, begs the question: what precipitated the event in the first place? These inconsistencies underscore the limitations of existing theories surrounding coronary artery involvement in MIs. As the spotlight shifts away from stable plaques, a pressing question emerges: What truly underlies the genesis of heart attacks? Unveiling the Autonomic Symphony: The Heart's Harmonious BalanceAn accurate understanding of myocardial ischemia necessitates consideration of the primary risk factors associated with heart disease, including gender, diabetes, smoking, and chronic psychological stress. Curiously, none of these risk factors directly implicate coronary artery pathology; instead, they impact capillary health or exert indirect effects. Over the past five decades, key medications in cardiology, such as beta-blockers, nitrates, aspirin, and statins, have demonstrated some benefits for heart patients. However, their mechanisms of action must be scrutinized within a comprehensive theory of myocardial ischemia. A groundbreaking revelation in heart disease prevention and treatment stems from the autonomic nervous system's role in ischemia genesis, as illuminated by heart-rate variability monitoring. The autonomic nervous system comprises two branches—the sympathetic and parasympathetic—responsible for regulating physiological responses. Imbalance between these branches emerges as a significant contributor to heart disease. Studies reveal a notable reduction in parasympathetic activity among patients with ischemic heart disease, particularly preceding ischemic events triggered by physical or emotional stressors. Conversely, abrupt increases in sympathetic activity rarely culminate in ischemia without antecedent parasympathetic decline. Notably, women exhibit stronger vagal activity than men, potentially influencing sex-based disparities in MI incidence. Multiple risk factors, including hypertension, smoking, diabetes, and stress, diminish parasympathetic activity, underscoring the pivotal role of the regenerative nervous system in heart health. Conversely, pharmacological interventions like nitrates, aspirin, and statins stimulate parasympathetic mediators, promoting ANS balance. In essence, while traditional risk factors and interventions influence plaque and stenosis development, their paramount impact lies in restoring ANS equilibrium. Thus, understanding the sequence of events leading to myocardial infarction demands a deeper exploration of autonomic nervous system dynamics. The Underlying pathophysiology of Myocardial IschemiaIn the vast majority of cases, the pathology leading to myocardial infarction (MI) begins with a decreased tonic activity of the parasympathetic nervous system (rest and digest), often exacerbated by physical or emotional stressors. This reduction prompts an increase in sympathetic nervous system activity, triggering heightened adrenaline production and directing myocardial cells to break down glucose via aerobic glycolysis, rather than their preferred fuel source of ketones and fatty acids (often explaining why patients report feeling tired before a MI). Remarkably, despite these metabolic shifts, no change in blood flow, as measured by the myocardial cell oxygen level (pO2), occurs. The shift towards glycolysis results in a surge of lactic acid production within myocardial cells, a phenomenon observed in nearly all MIs. This surge, coupled with localized tissue acidosis, impedes calcium entry into cells, compromising their contractility. Consequently, localized edema ensues, leading to hypokinesis—the hallmark of ischemic disease—and eventual tissue necrosis characteristic of an MI. Moreover, the ensuing tissue edema alters arterial hemodynamics, escalating sheer pressure and exacerbating plaque instability. This process elucidates the rupture of unstable plaques and their role in exacerbating arterial blockage during critical, acute scenarios. This explanation accounts for all the observable phenomena associated with heart disease. Understanding the etiology of heart disease holds profound implications beyond academic curiosity. It informs therapeutic strategies aimed at preserving parasympathetic activity, fostering holistic approaches to heart health, and challenging prevailing "civilized" industrial lifestyles. Central to this paradigm shift is the recognition of the vital role played by g-strophanthin—a hormone derived from the strophanthus plant. G-strophanthin is an endogenous hormone made in the adrenal cortex from cholesterol, whose production is inhibited by statin drugs, that does two things that are crucial for heart health and are done by no other medicine. G-strophanthin uniquely stimulates the production of acetylcholine, the primary neurotransmitter of the parasympathetic nervous system, while also converting lactic acid—the metabolic poison implicated in ischemic processes—into pyruvate, a preferred myocardial cell fuel. Perhaps this “magic” is why Chinese medicine practitioners say that the kidneys (i.e., adrenals, where ouabain is made) nourish the heart. Embracing this understanding not only guides therapeutic interventions but also underscores the imperative of dietary modifications. A diet abundant in healthful fats and fat-soluble nutrients, while low in processed carbohydrates and sugars, emerges as a cornerstone of heart health—a departure from the industrialized diets synonymous with modern civilization. In essence, unraveling the metabolic symphony orchestrating myocardial ischemia offers a transformative lens through which to perceive heart disease, fostering a holistic approach that transcends conventional paradigms and embraces the profound interconnectedness of mind, body, and environment. referencesGiorgio Baroldi. The Etiopathogenesis of Coronary Heart Disease. CRC Press EBooks, Informa, 20 Jan. 2004. Accessed 29 Mar. 2024.
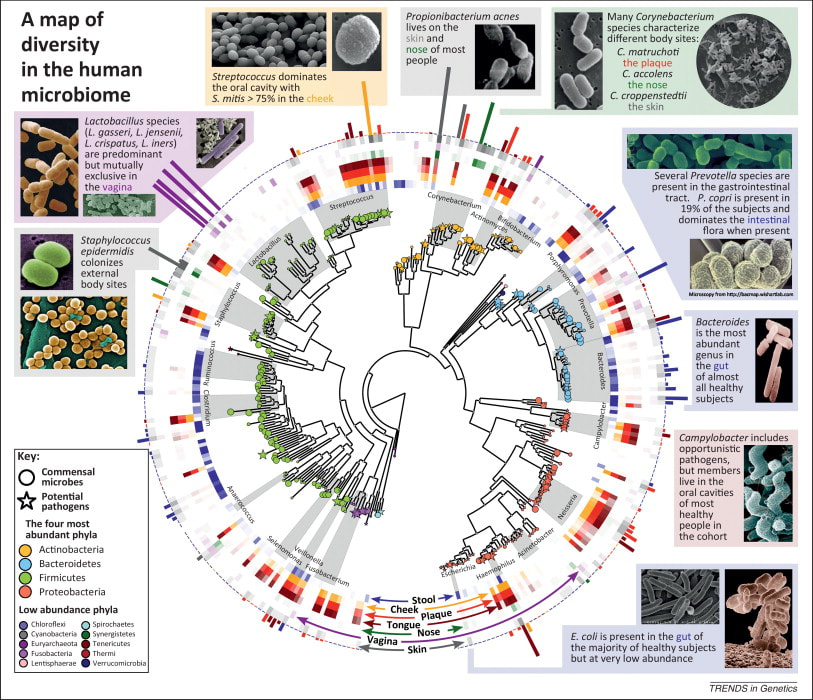
Sroka K. On the genesis of myocardial ischemia. Z Kardiol. 2004 Oct;93(10):768-83. doi: 10.1007/s00392-004-0137-6. PMID: 15492892. Helfant, R. H., et al. “Coronary Heart Disease. Differential Hemodynamic, Metabolic, and Electrocardiographic Effects in Subjects with and without Angina Pectoris during Atrial Pacing.” Circulation, vol. 42, no. 4, 1 Oct. 1970, pp. 601–610, www.ncbi.nlm.nih.gov/pubmed/11993303., https://doi.org/10.1161/01.cir.42.4.601. Takase, B., Kurita, A., Noritake, M., Uehata, A., Maruyama, T., Nagayoshi, H., ... & Nakamura, H. (1992). Heart rate variability in patients with diabetes mellitus, ischemic heart disease, and congestive heart failure. Journal of electrocardiology, 25(2), 79-88. Sroka, K., Peimann, C. J., & Seevers, H. (1997). Heart rate variability in myocardial ischemia during daily life. Journal of electrocardiology, 30(1), 45-56. Scheuer, J., & Brachfeld, N. (1966). Coronary insufficiency: relations between hemodynamic, electrical, and biochemical parameters. Circulation Research, 18(2), 178-189. Schmid, P. G., Greif, B. J., Lund, D. D., & Roskoski Jr, R. O. B. E. R. T. (1978). Regional choline acetyltransferase activity in the guinea pig heart. Circulation Research, 42(5), 657-660. Katz, A. M. (1971). Effects of ischemia on the cardiac contractile proteins. Cardiology, 56(1-6), 276-283. Manunta, Paolo, et al. “Endogenous Ouabain in Cardiovascular Function and Disease.” Journal of Hypertension, vol. 27, no. 1, 1 Jan. 2009, pp. 9–18, journals.lww.com/jhypertension/Abstract/2009/01000/Endogenous_ouabain_in_cardiovascular_function_and.3.aspx, https://doi.org/10.1097/HJH.0b013e32831cf2c6. Doepp, Manfred. “May Strophanthin Be a Valuable Cardiac Drug ? .” American Journal of Medical and Clinical Research & Reviews, vol. 2, no. 9, 15 Sept. 2023, pp. 1–6, ajmcrr.com/index.php/pub/article/view/75/74, https://doi.org/10.58372/2835-6276.1069. Accessed 29 Mar. 2024. Thayer, J. F., Yamamoto, S. S., & Brosschot, J. F. (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International journal of cardiology, 141(2), 122-131. Recent breakthrough studies have shone a light on the intriguing link between our microbiome – the diverse community of microorganisms residing in our gut and mouth – and the secret to a longer, healthier life. Scientists have long suspected that our genes, environment, and internal factors like the microbiome play a role in determining how long we live, but the specifics remained elusive. Now, thanks to cutting-edge research, we're getting closer to unraveling the mysteries of longevity. In this groundbreaking exploration, scientists employed a sophisticated approach called Mendelian randomization (MR) to delve into the intricate relationships between the human microbiome and longevity. By analyzing genetic data from large cohorts, they uncovered some compelling associations that shed light on the microbial players in the quest for a longer life. The Gut Chronicles: Microbial Superstars and CulpritsThe gut microbiome, a bustling metropolis of bacteria, has been a focal point in the quest for longevity. The study identified certain gut microbes as potential champions in the battle against aging. Microbial heroes like Coriobacteriaceae, Oxalobacter, and the probiotic Lactobacillus amylovorus were found to be positively linked to increased odds of longevity. On the flip side, a few gut microbes emerged as potential antagonists, with names like Fusobacterium nucleatum, Coprococcus, Streptococcus, Lactobacillus, and Neisseria negatively associated with longevity. These microbial foes might have a role in determining how gracefully we age. Oral Health: More Than Just a Pretty SmileThe study didn't stop at the gut; it extended its gaze to the oral microbiome, a less-explored but equally important realm. The findings suggested a fascinating connection between the oral microbiome and longevity. Specific oral bacteria were identified as potential influencers in the longevity game. Interestingly, the research hinted at a lower gut microbial diversity among centenarians (diversity appears to lower with age), but no significant difference in their oral microbiota. This finding underscores the importance of tracking the movements of these beneficial microbes across different parts of the body for a longer and healthier life. Decoding the Genetic Blueprint for LongevityThe study leveraged Mendelian randomization to unravel the causality between the microbiome and longevity. This approach, using genetic variants as tools, allowed scientists to explore the potential causal links between specific microbial features and the length of our lives. The bidirectional analyses provided a wealth of information, not only pinpointing specific microbes associated with longevity but also revealing the microbial preferences of genetically longevous individuals. For instance, genetic predisposition to longevity correlated with a higher abundance of Prevotella and a lower abundance of Bacteroides, suggesting a potential link between dietary choices and a longer life. Microbes and Diseases: Unraveling the We The study didn't just stop at longevity; it ventured into the realm of diseases. Certain microbes associated with longevity were found to have correlations with specific diseases. For example, Coriobacteriaceae, linked to longevity, was significantly reduced in patients with heart failure, suggesting a potential protective role against cardiovascular diseases. This "microbiota—disease—longevity" axis provides a nuanced understanding of how our microbial companions might influence not only our lifespan but also our susceptibility to various health conditions. What's Next in the Quest for a Longer LifeWhile the study opens exciting new avenues, there are some limitations to consider. The identified causalities didn't all reach statistical significance due to the vast number of microbial features tested. However, the robustness of the findings was supported by the replication of several identified causal links in independent datasets. Moving forward, researchers aim to collect more comprehensive individual-level data, including microbiome profiles, genetics, socio-economic factors, behaviors, and environmental influences. This holistic approach will help tease apart the individual contributions of these factors to longevity. In conclusion, this pioneering study, using Mendelian randomization, has provided us with a roadmap to explore the intricate connections between our microbiome and the quest for a longer, healthier life. As we unlock the secrets hidden in our genes and microbes, we inch closer to personalized approaches for healthy aging and interventions that could extend our time on this planet. referencesLiu, Xiaomin, et al. “Mendelian Randomization Analyses Reveal Causal Relationships between the Human Microbiome and Longevity.” Scientific Reports, vol. 13, no. 1, 29 Mar. 2023, p. 5127, www.nature.com/articles/s41598-023-31115-8, https://doi.org/10.1038/s41598-023-31115-8.
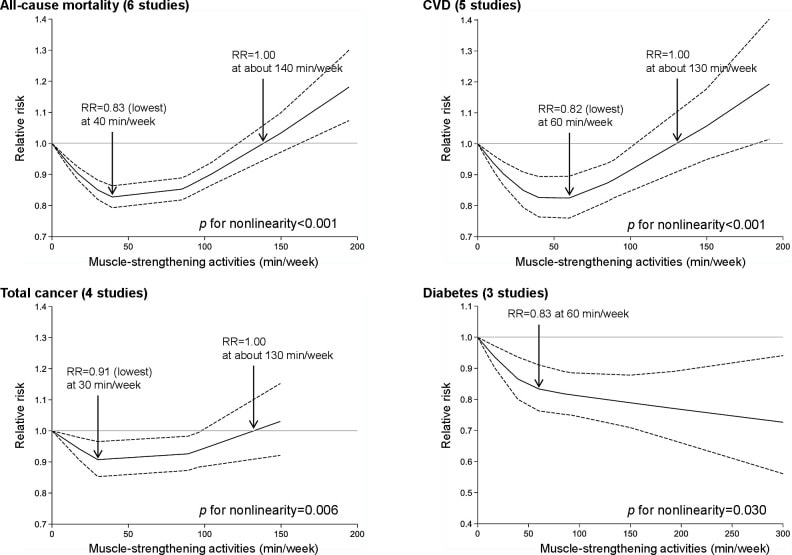
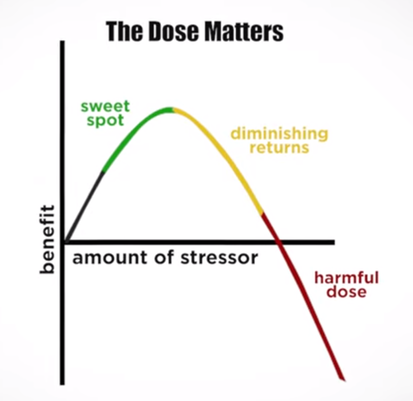
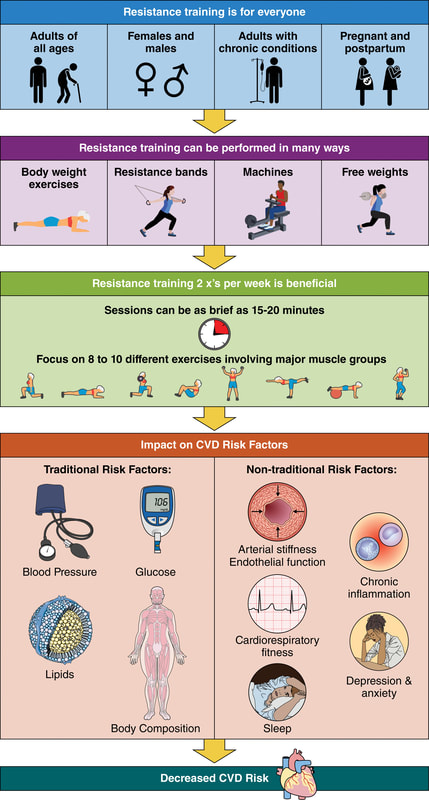
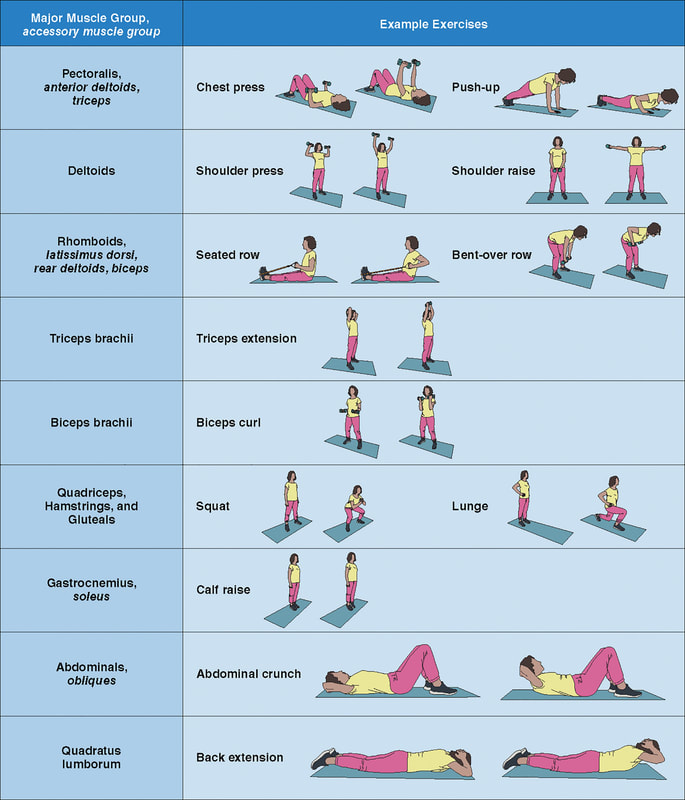
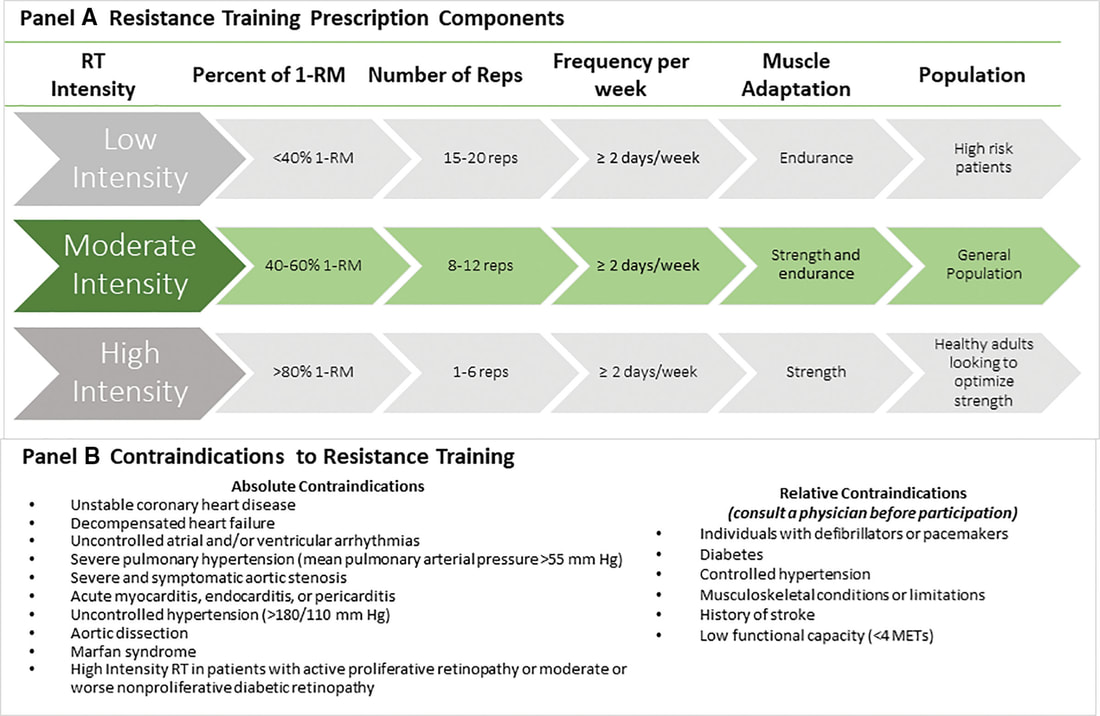
In an update to its 2007 scientific statement, the American Heart Association (AHA) emphasizes the significant and multifaceted benefits of resistance training (RT) on cardiovascular health. Contrary to the misconception that RT solely enhances muscle mass and strength, the statement highlights the favorable physiological and clinical effects of this form of exercise on cardiovascular disease (CVD) and associated risk factors. The scientific statement aims to provide comprehensive insights into the impact of RT, either alone or in combination with aerobic training, on traditional and nontraditional CVD risk factors. More is not always betterEpidemiological evidence suggests that RT is associated with a lower risk of all-cause mortality and CVD morbidity and mortality. Adults who participate in RT have ≈15% lower risk of all-cause mortality and 17% lower risk of CVD, compared with adults who report no RT. Approximately 30 to 60 minutes per week of RT is associated with the maximum risk reduction for all-cause mortality and incident CVD. Notice this "U" shape in the curve when examining the relationship between RT and morbidity and mortality. This curve suggests that some RT is clearly beneficial, but has the volume of RT increases past a certain point the benefits drop and it becomes harmful. The concept of a "biphasic response" is fundamental to understanding hormesis. It describes the characteristic dose-response relationship observed in hormetic processes, where a substance or stressor elicits opposite effects at low and high doses. The response can be visualized as a U-shaped or J-shaped curve, illustrating the beneficial effects at low doses and potential harm at higher doses. Benefits of RT on Traditional CVD Risk FactorsThe AHA's scientific statement underscores the positive influence of RT on traditional CVD risk factors, including blood pressure (BP), glycemia, lipid profiles, and body composition. Numerous studies indicate that engaging in RT is associated with reduced resting BP, improved glycemic control, and favorable alterations in lipid profiles, contributing to a lower risk of all-cause mortality and CVD morbidity. Despite recommendations suggesting 2 days per week of RT, only 28% of U.S. adults adhere to this guideline, highlighting the need for increased awareness and promotion. RT and resting blood pressureRT has demonstrated the ability to reduce resting BP across diverse populations, with notable benefits observed in individuals with prehypertension and hypertension. The mechanisms behind these benefits include enhancements in endothelial function, vasodilatory capacity, and vascular conductance. The reductions in BP achieved through RT are comparable to those achieved with antihypertensive medications. RT and GlycemiaRT shows promise in improving glycemia and insulin resistance, leading to a lower incidence of diabetes. The evidence suggests a nonlinear dose-response association, with up to 60 minutes per week of RT associated with the maximum risk reduction for diabetes. RT and Lipid ProfilesWhile the effect on lipid profiles is modest, RT results in favorable changes in high-density lipoprotein cholesterol, total cholesterol, and triglycerides. These improvements are more pronounced in older adults and those with elevated cardiometabolic risk. Rt, Body composition, and weightRT positively influences body composition by increasing lean body mass and reducing body fat percentage. It is particularly effective in overweight or obese individuals, contributing to increased metabolic rate and mitigating weight gain over time. Benefits of RT on Nontraditional CVD Risk FactorsIn addition to traditional risk factors, the scientific statement highlights the potential mechanisms by which RT positively affects nontraditional CVD risk factors. These include increased cardiorespiratory fitness, improved endothelial function, and potential benefits for sleep quality, psychological health, and well-being. The AHA's updated scientific statement reinforces the pivotal role of resistance training in cardiovascular health, providing a comprehensive overview of its impact on both traditional and nontraditional risk factors. As the evidence supporting RT's benefits continues to grow, the statement serves as a valuable resource for clinicians and public health professionals, offering practical strategies for promoting and prescribing resistance training to enhance cardiovascular health in diverse populations. ReferencesPaluch, Amanda E, et al. “Resistance Exercise Training in Individuals with and without Cardiovascular Disease: 2023 Update: A Scientific Statement from the American Heart Association.” Circulation, 7 Dec. 2023, https://doi.org/10.1161/cir.0000000000001189. Accessed 11 Dec. 2023.
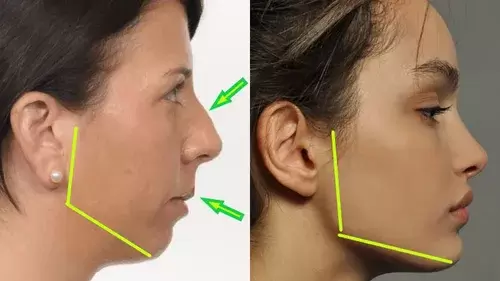
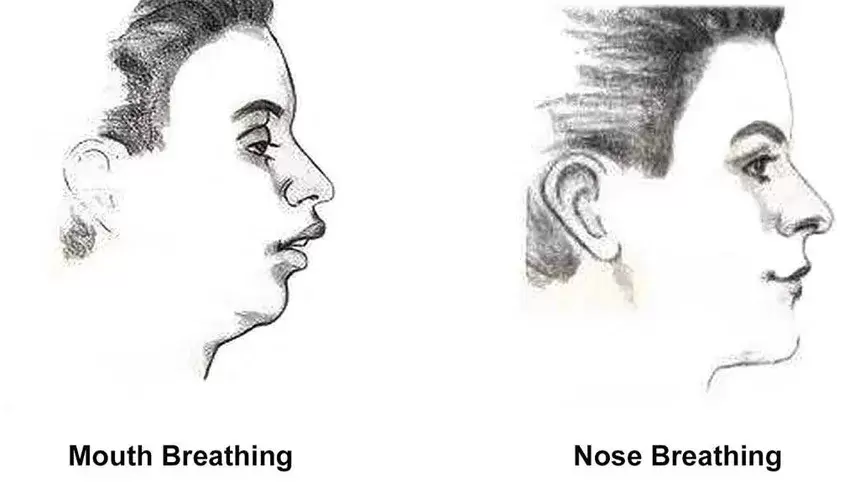
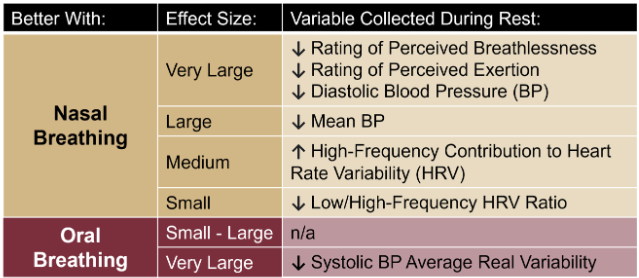
Momma H, Kawakami R, Honda T, Sawada SS. Muscle-strengthening activities are associated with lower risk and mortality in major non-communicable diseases: a systematic review and meta-analysis of cohort studies. Br J Sports Med. 2022 Jul;56(13):755-763. doi: 10.1136/bjsports-2021-105061. Epub 2022 Feb 28. PMID: 35228201; PMCID: PMC9209691. Nasal Breathing: A Breath of Fresh Air for Cardiovascular Wellness – Insights from New Research2/4/2024 The leading cause of death in the United States is cardiovascular disease, and the risk of cardiovascular issues can be predicted by factors such as blood pressure, heart rate variability, blood pressure variability, and cardiac vagal baroreflex sensitivity. The interplay between the cardiovascular and respiratory systems is highlighted, with a particular emphasis on how respiration affects key prognostic cardiovascular variables. This study explores the impact of nasal breathing compared to oral breathing on cardiovascular health in young adults. Nasal breathing is associated with humidification, warming, and filtration of inhaled air, potentially leading to bronchodilation and improved breathing efficiency. While past research has shown nasal breathing to have positive effects on resting metabolic demands, its influence on cardiovascular markers is not well-understood. The primary hypothesis is that nasal breathing, as opposed to oral breathing, will result in decreased blood pressure, improved heart rate variability, reduced blood pressure variability, and increased cardiac vagal baroreflex sensitivity at rest. The study aims to contribute to the understanding of how breathing patterns influence prognostic cardiovascular variables, aligning with the broader interest in the impact of breathing pace and training on cardiovascular health. The secondary hypothesis focuses on the effects of nasal breathing during submaximal exercise. The expectation is that nasal breathing, by attenuating the ventilatory response and metabolic demands, will lead to reduced blood pressure responses, improved heart rate variability, and decreased blood pressure variability during exercise. This aspect is particularly relevant due to the association between elevated exercise blood pressure and the risk of developing hypertension and cardiovascular disease. FindingsThe study findings are summarized, focusing on the impact of nasal vs. oral breathing on physiological and subjective variables at rest and during exercise. At rest, nasal breathing is associated with lower mean and diastolic blood pressure, improved heart rate variability metrics, reduced LF/HF ratio, and lower ratings of perceived exertion (RPE) and breathlessness (RPB). However, it increased systolic blood pressure average real variability. During submaximal exercise, differences between nasal and oral breathing were observed for RPB, suggesting a modest effect on reducing breathlessness during acute exercise. The discussion delves into the potential clinical significance of these findings, particularly the reduction in diastolic blood pressure during nasal breathing at rest. The study suggests a greater parasympathetic to sympathetic dominance during nasal breathing, indicated by changes in frequency-domain metrics of heart rate variability. Although nasal breathing did not significantly affect beat-to-beat blood pressure variability, there is speculation about potential connections between respiratory variables and blood pressure changes, emphasizing the need for further investigation. The study notes that the impact of nasal breathing on cardiovascular variables may have implications for various populations and suggests avenues for future research, including examining nasal breathing's effects on blood pressure over longer durations, both at rest and during activities like exercise. The discussion also touches on the potential benefits of interventions like mouth-taping overnight, emphasizing the importance of considering nasal breathing in the context of broader health outcomes. In summary, the study highlights the potential benefits of nasal breathing, with improvements in various cardiovascular and subjective measures at rest. While the effects during exercise are more modest, the findings contribute to understanding the nuanced relationship between respiratory patterns and cardiovascular health. referencesWatso, Joseph C., et al. “Acute Nasal Breathing Lowers Diastolic Blood Pressure and Increases Parasympathetic Contributions to Heart Rate Variability in Young Adults.” American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, vol. 325, no. 6, 1 Dec. 2023, pp. R797–R808, pubmed.ncbi.nlm.nih.gov/37867476/, https://doi.org/10.1152/ajpregu.00148.2023.
|
The Awareness domain contains research, news, information, observations, and ideas at the level of self in an effort to intellectualize health concepts.
The Lifestyle domain builds off intellectual concepts and offers practical applications.
Taking care of yourself is at the core of the other domains because the others depend on your health and wellness.
Archives
May 2024
Categories
All
|

 RSS Feed
RSS Feed

