|
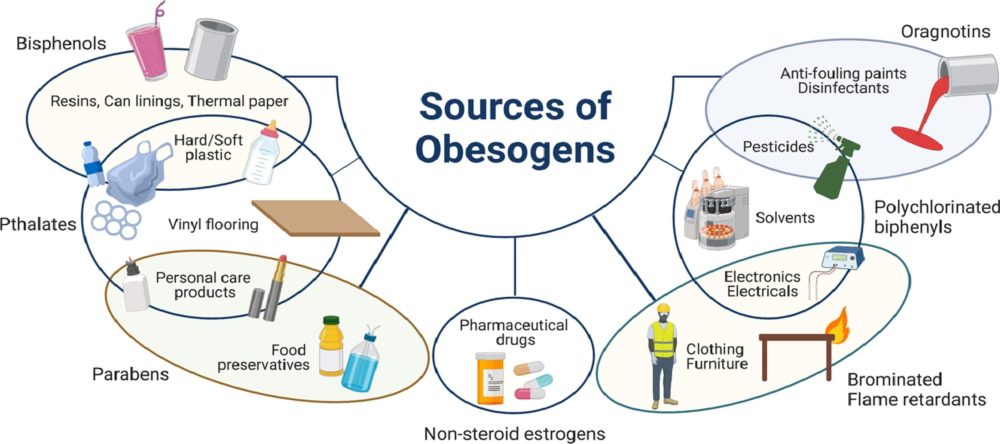
In our modern world, where convenience often comes at a cost, the prevalence of obesogens – chemicals that disrupt the body's normal metabolism and contribute to weight gain – has emerged as a growing concern. From everyday products to industrial pollutants, obesogens permeate our environment, exerting subtle yet profound effects on our health and well-being. Commonly encountered obesogensAmong the many obesogens encountered in daily life, several stand out for their widespread use and potential health impacts:
Mechanisms of ActionObesogens exert their effects through various mechanisms, including:
Disruption of Metabolism via MitochondriaObesogens, through their pervasive presence in our environment, exert insidious effects on metabolic function, including the intricate workings of mitochondria – the cellular powerhouses responsible for energy production. By disrupting mitochondrial function, obesogens can contribute to metabolic dysregulation and, ultimately, weight gain. Mitochondria play a central role in energy metabolism, converting nutrients into adenosine triphosphate (ATP), the primary source of cellular energy. However, exposure to obesogens can impair mitochondrial function through various mechanisms, including:
The disruption of mitochondrial function by obesogens can have profound implications for metabolic health and contribute to obesity through several pathways:
causative relationship with health conditionsThe impact of obesogens on human health extends beyond weight gain, with associations documented with various health conditions, including:
Additionally, obesogens are highly related to the following health conditions and physiologic imbalances:
Unraveling the Role of Dysfunctional Adipose TissueRelatively little is known about the extent to which obesogen exposure programs dysfunctional adipose tissue that may store but not mobilize fat. However, emerging evidence suggests that obesogens may contribute to adipocyte dysfunction, leading to altered fat storage and metabolism. One potential underlying factor is suboptimal liver detoxification pathways due to inadequate micronutrient cofactors. Inadequate levels of essential micronutrients, such as vitamins and minerals, can impair liver detoxification pathways responsible for metabolizing and eliminating obesogens from the body. As a result, obesogens may accumulate in adipose tissue, disrupting metabolic function and contributing to weight gain. Additionally, micronutrient deficiencies can compromise mitochondrial function, further exacerbating metabolic dysfunction and obesity risk. A Layman's Overview of Obesogens: Redefining the Weight Loss ParadigmIn the quest for weight loss, many of us often find ourselves fixating on calorie counting, fad diets, or intense workout regimens. However, what if I told you that the key to achieving a healthy weight isn't solely about shedding pounds but rather fixing your metabolism? Enter obesogens – a lesser-known yet influential factor in the obesity epidemic. As mentioned, obesogens are chemicals found in our environment, ranging from pesticides and plastics to food additives and personal care products. These substances have the uncanny ability to disrupt our body's natural weight-regulating mechanisms, leading to weight gain and metabolic dysfunction. Instead of solely blaming calories in versus calories out, it's essential to recognize the role obesogens play in shaping our metabolism. The Better Question: Fixing MetabolismRather than constantly asking ourselves, "How do I lose weight?" a more pertinent question would be: "How do I fix my metabolism?" Fixing metabolism involves addressing the root cause of weight gain – obesogen exposure and metabolic disruption. By eliminating or reducing our exposure to obesogens and ensuring our bodies receive essential micronutrients, we can optimize metabolic function and promote overall health. The Two-Fold SolutionTo achieve optimal health and maintain a healthy weight, a two-fold approach is necessary: 1. Reduce Toxin Exposure: Minimize exposure to obesogens by making conscious choices in our daily lives. This includes opting for organic produce, using natural cleaning and personal care products, and avoiding plastic containers and food packaging whenever possible. By participating in a structured evidenced-based detoxification program, we in turn lower our toxic burden, and we can mitigate the adverse effects of obesogens on our metabolism. 2. Consume Micronutrients: Vital micronutrients, such as vitamins and minerals, serve as essential cofactors in metabolic pathways. Ensuring adequate intake of these micronutrients through a balanced diet rich in fruits, vegetables, whole grains, and lean proteins can support optimal metabolic function. Additionally, supplementation may be necessary to address any deficiencies and promote metabolic health. The conventional approach to weight loss often overlooks the critical role obesogens play in metabolic dysfunction. Instead of solely focusing on calorie restriction or intense exercise, shifting our focus to fixing metabolism through toxin reduction and micronutrient consumption offers a more holistic and sustainable solution to achieving optimal health. By addressing the underlying factors contributing to metabolic disruption, we can pave the way for lasting weight management and overall well-being. the harm of environmental toxinsThe disruption of metabolic and mitochondrial function by obesogens represents a significant public health concern, with implications for obesity and metabolic disease. By understanding the mechanisms through which obesogens impair mitochondrial function and contribute to weight gain, researchers can develop targeted interventions to mitigate their adverse effects on metabolic health. Moreover, addressing underlying factors such as suboptimal liver detoxification pathways and micronutrient deficiencies is essential in combating the detrimental impact of obesogens on metabolic function and obesity prevalence. The pervasive presence of obesogens in our environment underscores the need for greater awareness and regulation of these harmful chemicals. By minimizing exposure to obesogens and advocating for safer alternatives, we can mitigate their adverse effects on human health and combat the rising tide of obesity and metabolic disease. As we navigate the complexities of modern living, vigilance and informed consumer choices are essential in safeguarding our health and well-being against the hidden threats of obesogens. Taking Action: The Integral Wellness ProgramFor those seeking tangible solutions to combat the effects of obesogens and improve their overall well-being, the Integral Wellness Program offers a comprehensive approach to optimizing health and vitality. This flagship service provides personalized guidance and support in key areas of movement, nutrition, and lifestyle to directly enhance quality of life. Online/In-Person Guidance One of the standout features of the Integral Wellness Program is its flexibility, offering both online and in-person consultations tailored to individual preferences and needs. Whether you prefer the convenience of virtual sessions or the hands-on approach of in-person coaching, our team of experienced wellness professionals is dedicated to supporting you every step of the way. Movement, Nutrition, and Lifestyle The Integral Wellness Program takes a holistic approach to health, addressing modifiable factors and behaviors in three core areas:
Augmenting the Health Process By participating in the Integral Wellness Program, you'll not only gain valuable knowledge and skills to navigate the challenges of modern living but also receive ongoing support and accountability to stay on track towards your health goals. Through targeted interventions aimed at eliminating obesogen exposure and promoting healthy behaviors, you can unlock your body's full potential and thrive in all aspects of life. The Integral Wellness Program offers a transformative journey towards optimal health and vitality. By prioritizing movement, nutrition, and lifestyle modifications, participants can take proactive steps to combat the effects of obesogens and reclaim control over their well-being. With the guidance and support of our dedicated wellness professionals, you'll embark on a path of self-discovery, empowerment, and lasting transformation. references
0 Comments
This article challenges the conventional understanding of heart disease, particularly the widely accepted theory that attributes its cause primarily to events occurring in the coronary arteries. Instead, a paradigm shift is proposed, contending that a deeper understanding of heart disease, encompassing angina, unstable angina, and myocardial infarction (heart attack), necessitates a focus on events within the myocardium, the muscular tissue of the heart. Over the past decades, the prevailing belief in the coronary artery theory has led to costly surgical interventions, widespread medication use with questionable benefits, and dietary recommendations that may exacerbate rather than alleviate the problem. By delving into the precise pathophysiological events that underlie heart attacks, we can uncover alternative approaches to prevention and treatment, such as adopting a "Nourishing Traditions"-style diet and utilizing safe and affordable medicines like g-strophanthin. Furthermore, this shift in perspective prompts us to confront broader issues, including the impact of modern lifestyles on human health, the need for a new medical paradigm, and the importance of ecological consciousness. Ultimately, reexamining the root causes of heart disease offers a pathway to addressing this pervasive health challenge and forging a healthier future for all. The information is summarized based on the work of Dr. Thomas Cowan, vice president of the Physicians Association for Anthroposophical Medicine and is a founding board member of the Weston A. Price Foundation. During his career he has studied and written about many subjects in medicine. These include nutrition, homeopathy, anthroposophical medicine, and herbal medicine. Challenging the Conventional model: Revisiting the Causes of Heart AttacksThe traditional understanding of heart attacks, largely centered on arterial blockage due to plaque buildup, has faced challenges in recent years. Initially, it was believed that blockages in the major coronary arteries led to oxygen deficiency in the heart, causing chest pain (angina) and eventually progressing to a heart attack. This simplistic view prompted invasive procedures like angioplasty, stents, and coronary bypass surgery as standard treatments. However, clinical observations and research findings have cast doubts on this approach. Anecdotal evidence (admittedly low quality evidence) from a trial in rural Alabama revealed surprising outcomes among individuals with single artery blockages. Contrary to expectations, less than 10% of those who experienced heart attacks did so in the region of the heart supplied by the blocked artery. Similarly, a comprehensive study conducted by the Mayo Clinic highlighted the limited efficacy of bypass surgery in preventing future heart attacks. While the procedure offered relief from chest pain, it did not significantly reduce the risk of subsequent heart events, except in high-risk patients. Contrary to popular belief, blockages exceeding 90% are often compensated for by collateral blood vessels, which develop over time to ensure uninterrupted blood flow to the heart. This extensive network of collateral vessels serves as a natural bypass system, mitigating the impact of arterial blockages on blood circulation. However, diagnostic procedures like coronary angiograms, which rely on injecting heavy dye into the arteries, often fail to accurately assess the extent of blockages and the true blood flow in the heart. As a result, many patients undergo invasive treatments such as bypass surgery, stents, or angioplasty based on misleading information about the severity of their arterial blockages. Moreover, studies have shown that these procedures provide minimal benefit, if any, to patients, particularly those with minimally symptomatic blockages exceeding 90%. Despite the widespread use of these interventions, their efficacy in restoring blood flow and preventing heart attacks remains questionable. These revelations underscore the need for a reevaluation of conventional treatment strategies and a deeper exploration of the underlying mechanisms behind heart attacks. Rather than focusing solely on arterial blockages, a more holistic approach that considers factors beyond plaque buildup may offer greater insights into the prevention and management of heart disease. Beyond the Coronary Artery TheoryThe prevailing focus in cardiology has long been on the stable, progressing plaque within the coronary arteries, deemed responsible for heart attacks. However, recent insights challenge this notion, redirecting attention to the unpredictable nature of unstable plaques. Unlike their calcified counterparts, unstable plaques are soft and prone to rapid evolution, abruptly occluding arteries and triggering downstream oxygen deficits, angina, and ischemia. These vulnerable plaques are believed to be a blend of inflammatory buildup and low-density lipoprotein (LDL), the primary targets of statin drugs. Consequently, the widespread adoption of statin therapy is advocated as a preventive measure against heart attacks, fueled by angiogram studies purportedly showcasing the prevalence of unstable plaques as the leading cause of myocardial infarctions (MIs). Yet, autopsies and pathology studies present a different narrative. Thrombosis, deemed crucial in precipitating MIs, is found in only a fraction of cases upon meticulous examination. Furthermore, measurements of myocardial oxygen levels during MIs reveal no discernible deficit, challenging the conventional understanding of ischemia as the primary mechanism. While thrombosis does occur in conjunction with MIs, its occurrence in less than half of cases underscores the inadequacy of attributing MIs solely to arterial blockages. The timing of thrombosis, often post-MI, begs the question: what precipitated the event in the first place? These inconsistencies underscore the limitations of existing theories surrounding coronary artery involvement in MIs. As the spotlight shifts away from stable plaques, a pressing question emerges: What truly underlies the genesis of heart attacks? Unveiling the Autonomic Symphony: The Heart's Harmonious BalanceAn accurate understanding of myocardial ischemia necessitates consideration of the primary risk factors associated with heart disease, including gender, diabetes, smoking, and chronic psychological stress. Curiously, none of these risk factors directly implicate coronary artery pathology; instead, they impact capillary health or exert indirect effects. Over the past five decades, key medications in cardiology, such as beta-blockers, nitrates, aspirin, and statins, have demonstrated some benefits for heart patients. However, their mechanisms of action must be scrutinized within a comprehensive theory of myocardial ischemia. A groundbreaking revelation in heart disease prevention and treatment stems from the autonomic nervous system's role in ischemia genesis, as illuminated by heart-rate variability monitoring. The autonomic nervous system comprises two branches—the sympathetic and parasympathetic—responsible for regulating physiological responses. Imbalance between these branches emerges as a significant contributor to heart disease. Studies reveal a notable reduction in parasympathetic activity among patients with ischemic heart disease, particularly preceding ischemic events triggered by physical or emotional stressors. Conversely, abrupt increases in sympathetic activity rarely culminate in ischemia without antecedent parasympathetic decline. Notably, women exhibit stronger vagal activity than men, potentially influencing sex-based disparities in MI incidence. Multiple risk factors, including hypertension, smoking, diabetes, and stress, diminish parasympathetic activity, underscoring the pivotal role of the regenerative nervous system in heart health. Conversely, pharmacological interventions like nitrates, aspirin, and statins stimulate parasympathetic mediators, promoting ANS balance. In essence, while traditional risk factors and interventions influence plaque and stenosis development, their paramount impact lies in restoring ANS equilibrium. Thus, understanding the sequence of events leading to myocardial infarction demands a deeper exploration of autonomic nervous system dynamics. The Underlying pathophysiology of Myocardial IschemiaIn the vast majority of cases, the pathology leading to myocardial infarction (MI) begins with a decreased tonic activity of the parasympathetic nervous system (rest and digest), often exacerbated by physical or emotional stressors. This reduction prompts an increase in sympathetic nervous system activity, triggering heightened adrenaline production and directing myocardial cells to break down glucose via aerobic glycolysis, rather than their preferred fuel source of ketones and fatty acids (often explaining why patients report feeling tired before a MI). Remarkably, despite these metabolic shifts, no change in blood flow, as measured by the myocardial cell oxygen level (pO2), occurs. The shift towards glycolysis results in a surge of lactic acid production within myocardial cells, a phenomenon observed in nearly all MIs. This surge, coupled with localized tissue acidosis, impedes calcium entry into cells, compromising their contractility. Consequently, localized edema ensues, leading to hypokinesis—the hallmark of ischemic disease—and eventual tissue necrosis characteristic of an MI. Moreover, the ensuing tissue edema alters arterial hemodynamics, escalating sheer pressure and exacerbating plaque instability. This process elucidates the rupture of unstable plaques and their role in exacerbating arterial blockage during critical, acute scenarios. This explanation accounts for all the observable phenomena associated with heart disease. Understanding the etiology of heart disease holds profound implications beyond academic curiosity. It informs therapeutic strategies aimed at preserving parasympathetic activity, fostering holistic approaches to heart health, and challenging prevailing "civilized" industrial lifestyles. Central to this paradigm shift is the recognition of the vital role played by g-strophanthin—a hormone derived from the strophanthus plant. G-strophanthin is an endogenous hormone made in the adrenal cortex from cholesterol, whose production is inhibited by statin drugs, that does two things that are crucial for heart health and are done by no other medicine. G-strophanthin uniquely stimulates the production of acetylcholine, the primary neurotransmitter of the parasympathetic nervous system, while also converting lactic acid—the metabolic poison implicated in ischemic processes—into pyruvate, a preferred myocardial cell fuel. Perhaps this “magic” is why Chinese medicine practitioners say that the kidneys (i.e., adrenals, where ouabain is made) nourish the heart. Embracing this understanding not only guides therapeutic interventions but also underscores the imperative of dietary modifications. A diet abundant in healthful fats and fat-soluble nutrients, while low in processed carbohydrates and sugars, emerges as a cornerstone of heart health—a departure from the industrialized diets synonymous with modern civilization. In essence, unraveling the metabolic symphony orchestrating myocardial ischemia offers a transformative lens through which to perceive heart disease, fostering a holistic approach that transcends conventional paradigms and embraces the profound interconnectedness of mind, body, and environment. referencesGiorgio Baroldi. The Etiopathogenesis of Coronary Heart Disease. CRC Press EBooks, Informa, 20 Jan. 2004. Accessed 29 Mar. 2024.
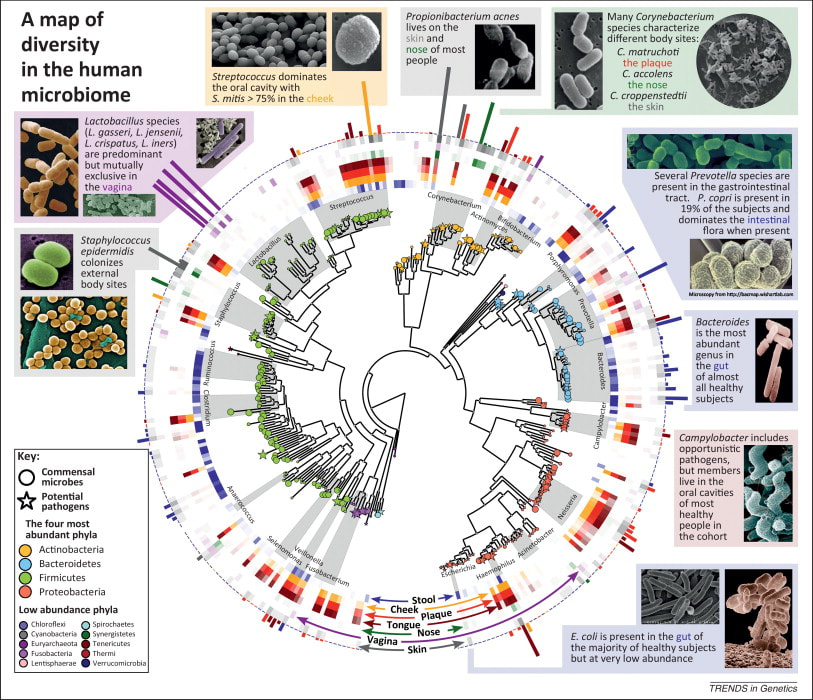
Sroka K. On the genesis of myocardial ischemia. Z Kardiol. 2004 Oct;93(10):768-83. doi: 10.1007/s00392-004-0137-6. PMID: 15492892. Helfant, R. H., et al. “Coronary Heart Disease. Differential Hemodynamic, Metabolic, and Electrocardiographic Effects in Subjects with and without Angina Pectoris during Atrial Pacing.” Circulation, vol. 42, no. 4, 1 Oct. 1970, pp. 601–610, www.ncbi.nlm.nih.gov/pubmed/11993303., https://doi.org/10.1161/01.cir.42.4.601. Takase, B., Kurita, A., Noritake, M., Uehata, A., Maruyama, T., Nagayoshi, H., ... & Nakamura, H. (1992). Heart rate variability in patients with diabetes mellitus, ischemic heart disease, and congestive heart failure. Journal of electrocardiology, 25(2), 79-88. Sroka, K., Peimann, C. J., & Seevers, H. (1997). Heart rate variability in myocardial ischemia during daily life. Journal of electrocardiology, 30(1), 45-56. Scheuer, J., & Brachfeld, N. (1966). Coronary insufficiency: relations between hemodynamic, electrical, and biochemical parameters. Circulation Research, 18(2), 178-189. Schmid, P. G., Greif, B. J., Lund, D. D., & Roskoski Jr, R. O. B. E. R. T. (1978). Regional choline acetyltransferase activity in the guinea pig heart. Circulation Research, 42(5), 657-660. Katz, A. M. (1971). Effects of ischemia on the cardiac contractile proteins. Cardiology, 56(1-6), 276-283. Manunta, Paolo, et al. “Endogenous Ouabain in Cardiovascular Function and Disease.” Journal of Hypertension, vol. 27, no. 1, 1 Jan. 2009, pp. 9–18, journals.lww.com/jhypertension/Abstract/2009/01000/Endogenous_ouabain_in_cardiovascular_function_and.3.aspx, https://doi.org/10.1097/HJH.0b013e32831cf2c6. Doepp, Manfred. “May Strophanthin Be a Valuable Cardiac Drug ? .” American Journal of Medical and Clinical Research & Reviews, vol. 2, no. 9, 15 Sept. 2023, pp. 1–6, ajmcrr.com/index.php/pub/article/view/75/74, https://doi.org/10.58372/2835-6276.1069. Accessed 29 Mar. 2024. Thayer, J. F., Yamamoto, S. S., & Brosschot, J. F. (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International journal of cardiology, 141(2), 122-131. Recent breakthrough studies have shone a light on the intriguing link between our microbiome – the diverse community of microorganisms residing in our gut and mouth – and the secret to a longer, healthier life. Scientists have long suspected that our genes, environment, and internal factors like the microbiome play a role in determining how long we live, but the specifics remained elusive. Now, thanks to cutting-edge research, we're getting closer to unraveling the mysteries of longevity. In this groundbreaking exploration, scientists employed a sophisticated approach called Mendelian randomization (MR) to delve into the intricate relationships between the human microbiome and longevity. By analyzing genetic data from large cohorts, they uncovered some compelling associations that shed light on the microbial players in the quest for a longer life. The Gut Chronicles: Microbial Superstars and CulpritsThe gut microbiome, a bustling metropolis of bacteria, has been a focal point in the quest for longevity. The study identified certain gut microbes as potential champions in the battle against aging. Microbial heroes like Coriobacteriaceae, Oxalobacter, and the probiotic Lactobacillus amylovorus were found to be positively linked to increased odds of longevity. On the flip side, a few gut microbes emerged as potential antagonists, with names like Fusobacterium nucleatum, Coprococcus, Streptococcus, Lactobacillus, and Neisseria negatively associated with longevity. These microbial foes might have a role in determining how gracefully we age. Oral Health: More Than Just a Pretty SmileThe study didn't stop at the gut; it extended its gaze to the oral microbiome, a less-explored but equally important realm. The findings suggested a fascinating connection between the oral microbiome and longevity. Specific oral bacteria were identified as potential influencers in the longevity game. Interestingly, the research hinted at a lower gut microbial diversity among centenarians (diversity appears to lower with age), but no significant difference in their oral microbiota. This finding underscores the importance of tracking the movements of these beneficial microbes across different parts of the body for a longer and healthier life. Decoding the Genetic Blueprint for LongevityThe study leveraged Mendelian randomization to unravel the causality between the microbiome and longevity. This approach, using genetic variants as tools, allowed scientists to explore the potential causal links between specific microbial features and the length of our lives. The bidirectional analyses provided a wealth of information, not only pinpointing specific microbes associated with longevity but also revealing the microbial preferences of genetically longevous individuals. For instance, genetic predisposition to longevity correlated with a higher abundance of Prevotella and a lower abundance of Bacteroides, suggesting a potential link between dietary choices and a longer life. Microbes and Diseases: Unraveling the We The study didn't just stop at longevity; it ventured into the realm of diseases. Certain microbes associated with longevity were found to have correlations with specific diseases. For example, Coriobacteriaceae, linked to longevity, was significantly reduced in patients with heart failure, suggesting a potential protective role against cardiovascular diseases. This "microbiota—disease—longevity" axis provides a nuanced understanding of how our microbial companions might influence not only our lifespan but also our susceptibility to various health conditions. What's Next in the Quest for a Longer LifeWhile the study opens exciting new avenues, there are some limitations to consider. The identified causalities didn't all reach statistical significance due to the vast number of microbial features tested. However, the robustness of the findings was supported by the replication of several identified causal links in independent datasets. Moving forward, researchers aim to collect more comprehensive individual-level data, including microbiome profiles, genetics, socio-economic factors, behaviors, and environmental influences. This holistic approach will help tease apart the individual contributions of these factors to longevity. In conclusion, this pioneering study, using Mendelian randomization, has provided us with a roadmap to explore the intricate connections between our microbiome and the quest for a longer, healthier life. As we unlock the secrets hidden in our genes and microbes, we inch closer to personalized approaches for healthy aging and interventions that could extend our time on this planet. referencesLiu, Xiaomin, et al. “Mendelian Randomization Analyses Reveal Causal Relationships between the Human Microbiome and Longevity.” Scientific Reports, vol. 13, no. 1, 29 Mar. 2023, p. 5127, www.nature.com/articles/s41598-023-31115-8, https://doi.org/10.1038/s41598-023-31115-8.
|
The Awareness domain contains research, news, information, observations, and ideas at the level of self in an effort to intellectualize health concepts.
The Lifestyle domain builds off intellectual concepts and offers practical applications.
Taking care of yourself is at the core of the other domains because the others depend on your health and wellness.
Archives
May 2024
Categories
All
|


 RSS Feed
RSS Feed

