|
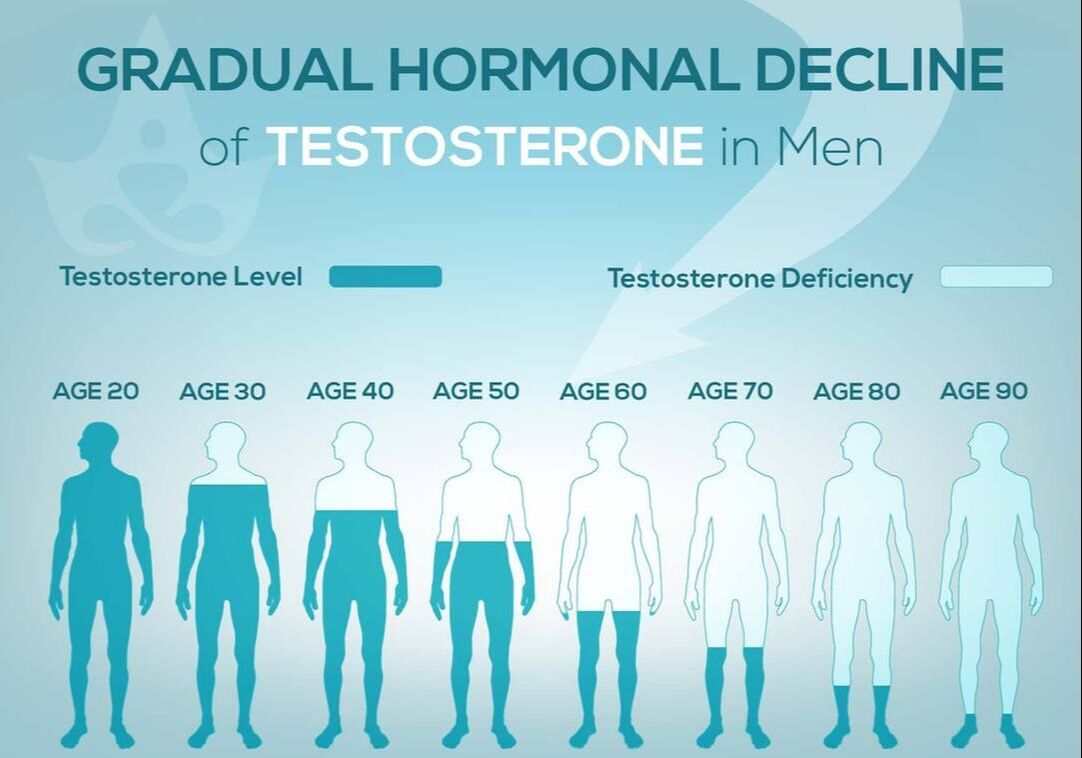
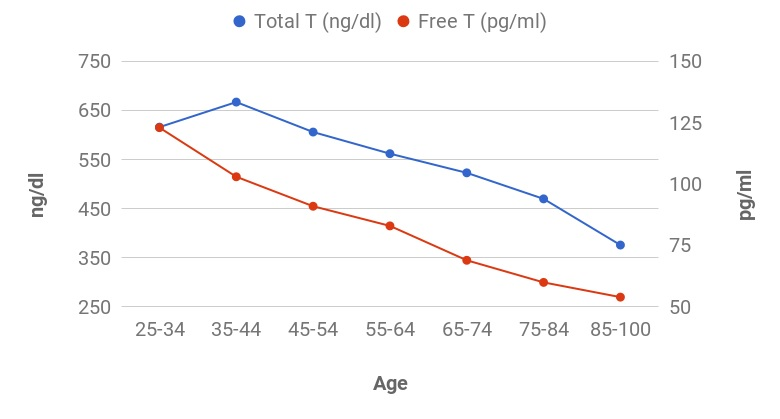
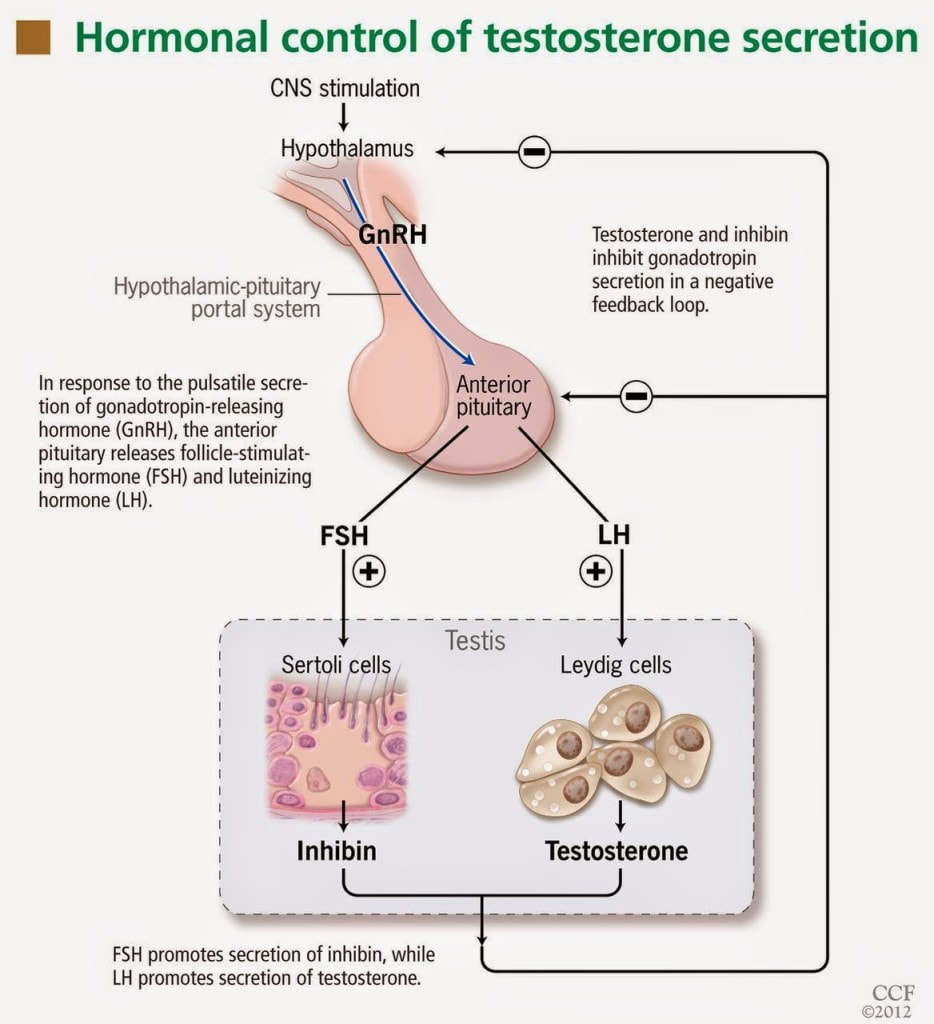
Women are not the only ones who experience hormonal changes in midlife. Men also undergo a transition similar to menopause called andropause, also known as late-onset hypogonadism (LOH), during which vitality hormones, particularly testosterone (T), but also human growth hormone (HGH), are produced in lower quantities. As men age, not only does the body start making less testosterone, but also the levels of another hormone called sex hormone binding globulin (SHBG), which pulls usable testosterone from the blood, begins to increase. Andropause is a natural phase of aging, but is accentuated by many factors including, but not limited to age-associated comorbid illnesses, medications, and malnutrition. The age at which symptoms of andropause may manifest can vary, but it typically occurs in middle-aged and older men, beginning in the late 40s to early 50s, with the most frequent age occurring between 51-60 years, with patients reporting symptoms such as impotence, weakness, and memory loss. Other age-related alterations due to andropause include body composition, mood, cognitive function, sleep, and erythropoiesis. The pharmaceutical industry has capitalized heavily on this 'change of life' phase, with Viagra, among other pharmaceuticals, but these pharmaceuticals have severe, if not sometimes deadly side effects. All the more reason why modifiable factors and natural alternatives are in great need today. While the process of andropause is considered inevitable, understanding the causes and adopting proactive nutrition and lifestyle strategies can prevent an early onset of this condition, and significantly alleviate symptoms thereby enhancing overall quality of life. normalization of Low TTestosterone is a crucial hormone for both men and women, contributing to muscle bulk, strength, fat-burning, and overall vitality. Adequate testosterone levels provide a chiseled look, high energy, and strength in men, and definition, muscle, and energy in women. However, declining testosterone levels can lead to increased fatigue, difficulty building muscle, and higher fat accumulation, posing a risk to overall health. Contrary to the common belief that decreasing testosterone is solely an aging-related issue, it has been observed that testosterone levels, even in young men, have been declining for decades. Factors contributing to this decline include the Standard American Diet, characterized by high sugar intake, imbalanced omega-6 to omega-3 ratios, processed foods lacking essential nutrients, and the attack on cholesterol. Additionally, environmental toxins play a role in lowering testosterone levels. Notably, the Standard American Diet, abundant in sugars, particularly processed sugars and carbohydrates, elevates cortisol levels, which inversely affects testosterone. The imbalance of omega-6 to omega-3 ratios, primarily due to the prevalence of corn and soy in processed foods, further contributes to cortisol elevation, adversely impacting testosterone levels. Processed foods with low nutritional value, combined with the demonization of cholesterol, essential for testosterone production, also play a role in this decline. Addressing these issues through dietary adjustments, such as choosing organic and grass-fed options, avoiding processed foods and sugars, ensuring adequate protein intake, and engaging in regular exercise, can positively impact testosterone levels and overall health. Understanding and addressing these lifestyle factors is essential for maintaining optimal hormonal balance and supporting longevity. PSYCHONEUROIMMUNOLOGY: |
Acid Blockers |
ADHD drugs |
Adjuvant |
Adderall |
Adrenaline |
Aluminum |
Antidepressants |
Antihypertensive drugs |
Antipsychotic drugs |
Antiretroviral drugs |
Atorvastatin |
Benzophenones |
Bile Acid Sequestrants (+ binding resins) |
Bisphenols (BPA, BPF, BPS) |
Cell Phone Exposure |
Cesium-137 |
Cholesterol Lowering Drugs |
Concerta |
Corticosteroid |
Dexamethasone |
Electronic Cigarettes |
Ethinyl Estradiol (plus Lynestrenol) |
Ethylene Glycol |
Fluoride |
Fructose |
Gluten (and Exorphins) |
Hexachlorocyclohexane |
Histamine Receptor Antagonists |
Ibuprofen |
Infant Formula |
Lead |
Levonorgestrel/ethinyl estradiol |
Lovastatin |
Mercury |
Monosodium Glutamate (MSG) |
Mycoestrogens |
Nanoparticles |
Nonylphenol [and Ethoxylate (NPE)] |
Oral Contraceptives |
Organochlorine Pesticides & Compounds |
Organophosphate Pesticides |
Persistent Organic Pollutants (POPs) |
Pesticides |
Phenothrin |
Polybrominated Diphenylethers (PBDEs) |
Polyoxyethylene Amine |
Prednisone |
Pravastatin |
Progestins |
Rosuvastatin |
Simvastatin |
Sodium Fluoride |
Soy |
Statin Drugs |
Sugar Sweetened Beverages |
Tamoxifen |
Thimerosal |
Thiazide Diuretics |
Tin |
Titanium Nanoparticles (including Dioxide) |
Tween 80 (Polysorbate 80) |
Vinclozolin |
Vitamin A Palmitate |
Zearalenone (ZEA) |
It's important to be aware of potential exposure to these substances and take steps to minimize risks. This includes choosing products that are labeled as BPA-free, using natural and organic personal care products, and being mindful of pesticide residues on food. Additionally, maintaining a healthy lifestyle, including a balanced diet and regular exercise, can help support overall endocrine health.
Bisphenol-A (BPA)
The mechanism by which BPA may lead to lower testosterone levels involves its ability to mimic or interfere with the action of hormones. BPA is known to have estrogenic properties, meaning it can bind to estrogen receptors in the body, thereby mimicking the effects of estrogen. This can disrupt the delicate hormonal balance, leading to alterations in the normal regulatory processes of the endocrine system.
Excessive estrogenic activity, whether from BPA or other sources, can negatively impact the production of testosterone. Estrogen and testosterone are usually balanced in the body, and disruptions in this balance can lead to a decrease in testosterone levels. BPA may interfere with the function of Leydig cells in the testes, which are responsible for producing testosterone. This interference can result in reduced testosterone synthesis.
BPA may interfere with the signaling pathways involved in hormone production and regulation. This disruption can lead to a cascade of effects, including reduced stimulation of testosterone production by luteinizing hormone (LH) from the pituitary gland.
BPA exposure has been associated with testicular abnormalities, including changes in testicular morphology and function. These changes can contribute to lower testosterone levels.
It's important to note that the impact of BPA on testosterone levels can be influenced by factors such as the duration and level of exposure, individual sensitivity, and overall health. Chronic exposure to BPA, particularly during critical developmental periods, may have more pronounced effects. Reducing exposure to BPA by using BPA-free products, choosing fresh foods over canned goods, and being mindful of plastic usage may help mitigate potential risks.
Smoking
Leydig cells in the testes are responsible for producing testosterone. Smoking exposes the body to various harmful chemicals, including those in cigarette smoke, and glycerin. These toxic substances can directly affect Leydig cells, leading to dysfunction and a decrease in testosterone production.
Smoking generates oxidative stress in the body due to the production of free radicals and reactive oxygen species. Oxidative stress has been linked to damage to testicular cells, including Leydig cells. This damage can interfere with the normal process of testosterone synthesis.
Smoking is known to constrict blood vessels and impair blood flow. This vasoconstriction can affect blood supply to the testes, compromising their function. Inadequate blood flow to the testes may contribute to decreased testosterone production.
Smoking can disrupt the delicate balance of hormones involved in reproductive health. For example, it may lead to an increase in cortisol, a stress hormone, which can negatively influence testosterone levels. Hormonal imbalances, particularly elevated stress hormones, can interfere with the normal regulatory mechanisms of testosterone production.
Smoking has been associated with increased aromatase activity. As mentioned, higher aromatase activity can lead to a greater conversion of testosterone to estrogen, resulting in lower testosterone levels.
Smoking has been linked to structural damage in the testes. This damage can impact the overall health of testicular tissue and contribute to lower testosterone levels.
Luteinizing hormone (LH) stimulates the production of testosterone by the Leydig cells. Smoking has been associated with decreased levels of LH. Reduced LH levels can result in diminished stimulation of testosterone production.
It's important to note that the impact of smoking on testosterone levels can vary among individuals, and factors such as the duration and intensity of smoking, overall health, and genetic predisposition may influence the extent of the effect. Quitting smoking is a crucial step in promoting overall health, including reproductive health, and may contribute to the restoration of normal testosterone levels over time.
Alcohol
Chronic alcohol consumption has been linked to testicular atrophy, which is a reduction in the size and function of the testes. Testicular atrophy may result in a decreased ability of Leydig cells (which produce and respond to testosterone) to function optimally, leading to lower testosterone levels.
Alcohol can disrupt the normal hormonal regulation of the endocrine system. Chronic alcohol use may alter the balance of hormones involved in reproductive health. Alcohol consumption has been associated with increased cortisol levels (a stress hormone), which can have inhibitory effects on testosterone production.
Alcohol can suppress the release of GnRH, a hormone that stimulates the production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary gland. Reduced GnRH levels may lead to lower LH levels, which, in turn, can diminish the stimulation of testosterone production by Leydig cells.
Chronic alcohol consumption has been associated with increased aromatase activity. Elevated aromatase activity can lead to a greater conversion of testosterone to estrogen, resulting in lower testosterone levels.
The liver is involved in the metabolism of hormones, including testosterone. Chronic alcohol use can lead to liver damage and impaired liver function. Liver dysfunction may impact the normal clearance and metabolism of hormones, potentially contributing to hormonal imbalances, including lower testosterone levels.
Alcohol interferes with the absorption and utilization of certain nutrients, including zinc. Zinc is an essential mineral for testosterone production. Nutrient deficiencies, particularly zinc deficiency, may contribute to decreased testosterone synthesis.
Excessive alcohol consumption can disrupt sleep patterns. Sleep is crucial for the natural production of testosterone during the night. Poor sleep quality or insufficient sleep may negatively impact testosterone levels.
It's important to note that the impact of alcohol on testosterone levels can vary among individuals, and factors such as the amount and duration of alcohol consumption, overall health, and genetic predisposition may influence the extent of the effect. Moderation in alcohol consumption and maintaining a healthy lifestyle are important considerations for overall well-being, including reproductive health.
Excess Environmental Heat (sauna)
- Improved Circulation and Blood Flow: Improved blood flow may enhance nutrient and oxygen delivery to tissues, including the testes, potentially supporting optimal Leydig cell function responsible for testosterone production.
- Heat Stress and Hormetic Response: Hormetic stressors, including heat stress from sauna use, may stimulate the release of certain hormones, potentially influencing the endocrine system, including testosterone regulation.
- Stress Reduction and Cortisol Modulation: Chronic stress and elevated cortisol levels have been linked to disruptions in testosterone balance. Sauna-induced relaxation may help modulate cortisol levels and support hormonal balance.
- Detoxification and Elimination of Toxins: Some environmental toxins may interfere with hormone balance, and the elimination of these toxins through the skin may indirectly support hormonal health, including testosterone levels.
- Enhanced Recovery and Exercise Benefits: Regular exercise is associated with improved testosterone levels. Sauna acts as a non-impact cardio sessions, and especially when performed post-exercise may enhance recovery, potentially supporting the overall positive effects of exercise on hormonal health.
- Improved Sleep Quality: Sauna use, particularly in the evening, has been reported to promote relaxation and improve sleep quality. Quality sleep is crucial for the natural regulation of hormones, including testosterone. Improved sleep may indirectly contribute to hormonal balance.
- Cardiovascular Health Benefits: Sauna use has been associated with cardiovascular benefits, including improved endothelial function and reduced blood pressure. Cardiovascular health is linked to overall well-being, and maintaining a healthy cardiovascular system may positively influence hormonal balance.
Given the notable benefits of sauna, it has been demonstrated that prolonged exposure to excessive heat, such as in hot environments or the use of hot baths and saunas, has been associated with potential impairment of testosterone levels. Several mechanisms may contribute to the negative impact of heat on testosterone.
The testes are located outside the body in the scrotum, a sac of skin. This positioning is crucial for maintaining a lower temperature than the core body temperature, which is necessary for optimal sperm and testosterone production. Prolonged exposure to heat, especially if the testes are subjected to elevated temperatures, can disrupt the normal temperature regulation and impair the function of Leydig cells, which produce testosterone.
Elevated testicular temperatures resulting from prolonged exposure to heat have been linked to a decrease in sperm production (spermatogenesis). The same conditions that inhibit spermatogenesis may also impact Leydig cell function, leading to a reduction in testosterone synthesis.
GnRH stimulates the release of luteinizing hormone LH from the pituitary gland. LH, in turn, stimulates the Leydig cells to produce testosterone. Prolonged heat exposure has been associated with a decrease in GnRH and LH levels, potentially leading to reduced stimulation of testosterone production.
The body perceives excessive heat as a stressor, leading to the activation of the stress response, including the release of cortisol. Elevated cortisol levels can negatively impact the balance of sex hormones, potentially suppressing testosterone synthesis. While sauna has been observed to lower cortisol, perception and experience are important to note. In other words, if an individual does not have much experience with deliberate heat exposure and decides to enter extreme heat for long durations, their body likely cannot compensate to the stressor. Gradual progressions in intensity and durations for sauna use are recommended.
High temperatures can affect the quality of sperm, leading to decreased motility and fertility. The relationship between impaired sperm quality and testosterone levels suggests that the detrimental effects of heat may extend to Leydig cell function and testosterone synthesis.
It's important to note that the impact of heat on testosterone levels can vary among individuals, and the body's ability to regulate temperature may differ. Additionally, the body has mechanisms to cope with short-term variations in temperature. However, chronic or extreme heat exposure may pose risks to reproductive health.
Non-native EMFs
A comprehensive review of studies from 2003 to 2020 highlighted several key findings:
- Sperm Quality: Both human and animal studies indicate that exposure to EMR from mobile phones leads to reduced sperm motility, structural anomalies, and increased oxidative stress due to overproduction of reactive oxygen species (ROS).
- Semen Analysis: Human semen samples exposed to mobile phone EMR showed significant reductions in motility and viability, and increased ROS levels. Laboratory-controlled exposure of semen samples to mobile phone radiation resulted in decreased sperm concentration and quality.
- Epidemiological Studies: Research involving large cohorts of men demonstrated a correlation between mobile phone usage and lower semen volume, sperm concentration, and total sperm count. Constant use of mobile internet services was particularly linked to poorer sperm quality.
- Survey Results: Surveys of men referred for semen analysis revealed that prolonged phone usage (over an hour per day) and usage while charging were associated with higher percentages of abnormal sperm concentrations.
- Experimental Findings: Studies on male rats exposed to mobile phone radiation showed slight decreases in serum testosterone levels and testicular weight. Other experiments demonstrated that EMR exposure led to genotoxic effects on spermatozoa and altered pituitary function, affecting the Leydig and Sertoli cells critical for male fertility.
- Laptop Exposure: Sperm samples exposed to Wi-Fi radiation from laptops for extended periods showed reduced motility and increased DNA fragmentation.
- Military Study: An increased rate of childlessness was observed among military men exposed to RF electromagnetic fields (EMF), further supporting the link between EMR exposure and male infertility.
Here are some proposed mechanisms through which exposure to non-native EMFs, such as Wi-Fi, Bluetooth, cellphones, and sources of "dirty electricity" including electric-generated heaters, negatively influence testosterone levels:
- Scrotal Hyperthermia and Oxidative Stress: These were identified as primary mechanisms through which EMR affects male fertility. Long-term and frequent use of mobile phones exacerbates these effects.
- Increased Oxidative Stress: Exposure to EMFs has been associated with increased oxidative stress in some studies. Oxidative stress refers to an imbalance between free radicals and antioxidants in the body. Elevated oxidative stress may have the potential to disrupt the endocrine system, including the regulation of testosterone.
- Disruption of Melatonin Production: EMF exposure, especially from devices used at night like cellphones, may interfere with melatonin production. Melatonin is a hormone that regulates sleep-wake cycles. Disruption of melatonin levels can impact the circadian rhythm and potentially influence testosterone production, as testosterone follows a circadian pattern with higher levels during sleep.
- Alteration of Calcium Ion Movement: Researchers have observed that non-native EMFs increase the movement of calcium ions in cells. Calcium ions play a role in various cellular processes, including hormone production. Disruption of calcium ion movement influences signaling pathways involved in testosterone synthesis, among other harmful effects.
- Impact on Leydig Cells: As mentioned, Leydig cells in the testes are responsible for producing testosterone. Researchers have observed exposure to EMFs affects Leydig cell function, thereby altering testosterone synthesis.
- Heat Generation: Certain devices emitting EMFs, especially those with high power, may generate heat. Prolonged exposure to localized heat, particularly in the groin area where the testes are located, could potentially impact sperm quality and testosterone production
Environmental Toxins
- Endocrine Disruption: Many environmental toxins are classified as endocrine-disrupting chemicals (EDCs). These substances can mimic or interfere with the actions of hormones, including testosterone. EDCs may bind to hormone receptors, blocking or activating them inappropriately. This interference can lead to imbalances in hormonal signaling, including the regulation of testosterone production.
- Aromatase Activity and Estrogen Dominance
- Disruption of Leydig Cell Function
- Inhibition of Gonadotropins: Some environmental toxins can interfere with the secretion of gonadotropins, such as LH and FSH, which regulate testosterone production. Inhibition of gonadotropin release may lead to diminished stimulation of Leydig cells and, consequently, lower testosterone levels.
- Testicular Toxicity: Certain environmental toxins may exhibit testicular toxicity, causing damage to the testes and impairing their function. Testicular damage can impact Leydig cell activity and overall testosterone synthesis.
- Oxidative Stress: Some environmental toxins can induce oxidative stress, resulting in an imbalance between free radicals and antioxidants in the body. Oxidative stress has been associated with testicular damage and impaired testosterone production.
- Impaired Sperm Quality: Environmental toxins may affect sperm quality, including motility and morphology. Sperm abnormalities can be indicative of disruptions in the testicular microenvironment, potentially influencing testosterone levels.
- Epigenetic Changes: Exposure to environmental toxins may lead to epigenetic changes, alterations in gene expression without changes to the underlying DNA sequence. Epigenetic modifications in genes related to testosterone synthesis and regulation can impact hormonal balance.
Below is a list of known compounds, chemicals, and environmental toxins that reduce testosterone levels, as supported by scientific literature:
Anti-Androgens |
Atorvastatin |
Bisphenol A |
Ethinyl Estradiol (plus Lynestrenol) |
Glyphosate (Roundup |
Ibuprofen |
Levonorgestrel / ethinyl estradiol |
Lovastatin |
Organophosphate pesticides |
Parabens |
Pesticides |
Phthalates |
Simvastatin |
Statin Drugs |
Sugar Sweetened Beverages |
Titanium Dioxide |
Titanium Nanoparticles |
Vitamin A Palmitate |
Solutions to andropause
It's important to note that individual responses to lifestyle interventions can vary, and results may take time.
Nutritional Strategies
Here is an evidence-based list of foods, compounds, and substances known to enhance testosterone levels:
Astragalus |
Astaxanthin |
Bitter Melon |
Biochanin A |
Caffeine |
Calcium* |
Coconut (+ Oil) |
Coleus Forskohlii |
Curcumin |
Daidzein |
Dogwood |
Fermented Foods and Beverages |
Formononetin |
Genistein |
|
Ginseng (Korean) |
Ginsenosides |
Ginkgo biloba |
Isoflavones |
Linoleic acid^ (Conjugated) |
Maca |
Molybdenum |
Mulberry |
||
Olive |
Onion |
Pantothenic Acid (Vitamin B-5) |
Phosphatidylserine |
Phytoestrogens (+/-) |
Raspberry |
Resveratrol |
Saffron |
Selenium |
|
Squalene |
Suma (Pfaffia Paniculata) |
Taro |
Tauroursodeoxycholic acid |
|
Vitamin E |
Zinc |
Before embarking on a supplement regimen, prioritize the quality of ingredients. Opt for reputable brands that use high-quality, pure ingredients. The effectiveness and safety of a supplement are inherently linked to the quality of the components it contains.
Understanding the appropriate dosage for each compound is paramount. Dosage recommendations can vary based on factors such as age, health status, and individual response. Always follow recommended dosages and, if uncertain, consult with a healthcare professional for personalized advice.
Enhancing testosterone is not just about isolated compounds; it's a holistic journey. Lifestyle factors, including nutrition, exercise, sleep, and stress management, play pivotal roles in hormonal balance. Consider adopting a well-rounded approach that encompasses these lifestyle elements.
Before introducing additional compounds, it's wise to address and minimize harmful stressors in your life. Chronic stress, inadequate sleep, and poor dietary choices can negatively impact hormone levels. Individuals may find more significant benefits by first focusing on stress reduction and overall well-being.
It's essential to recognize that responses to supplements can vary widely among individuals. What works for one person may not yield the same results for another. Pay attention to how your body responds, and be patient; changes may take time.
*^The hormonal system is complex and nuanced. Just because researchers have observed the calcium and linoleic acid (LA) can increase testosterone, more is not better. Excess LA (an essential fatty acid - the body cannot make it, and must consume it in the diet) is well established to disrupt metabolic function, which can thereby lead to lower testosterone. Innumerable amounts of food contain LA, therefore to call it "essential" can be deceiving. It is important to note that both calcium and LA should be consumed in whole food sources.
In conclusion, while compounds like zinc, magnesium, and various herbs have been associated with potential testosterone support, a thoughtful and informed approach is crucial. Prioritize high-quality ingredients, determine suitable dosages, and consider the broader lifestyle factors influencing hormonal health. Taking proactive steps to reduce harmful stressors can set a solid foundation for any testosterone-enhancing efforts. Before making significant changes to your supplement routine, it's advisable to consult with healthcare professionals for personalized guidance tailored to your unique needs. Remember, optimizing testosterone is a holistic endeavor that encompasses both supplementation and a balanced, healthy lifestyle.
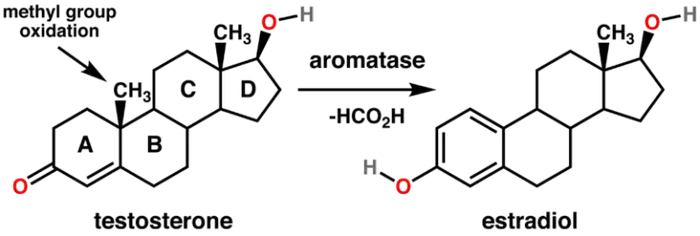
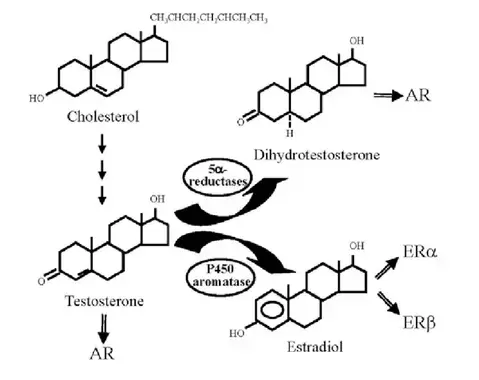
Aromatase inhibitors
Aromatase inhibitors are often utilized in medical scenarios where reducing estrogen levels is crucial, such as in the treatment of hormone-sensitive breast cancer in postmenopausal women. In men undergoing TRT, aromatase inhibitors may be used to manage or prevent symptoms of estrogen dominance that can occur with exogenous testosterone administration. Aromatase inhibitors should be used judiciously, as completely suppressing estrogen levels in men can have adverse effects on bone health, libido, and overall well-being. Additionally, as paradoxical as it might sound, some aromatase inhibitors are actually estrogenic (e.g. anastrozole). The use of aromatase inhibitors should be tailored to individual needs, and regular monitoring of hormone levels is essential to ensure a balanced hormonal profile.
There are many natural aromatase inhibitors including progesterone, Maca, Grape seed extract, Nettle, Saw Palmetto, and more. The following foods contain compounds that have been shown to inhibit aromatase activity, thereby suppressing estrogen biosynthesis:
In summary, aromatization is a natural and complex process with essential physiological functions. However, imbalances in aromatase activity can have implications for hormonal health. Aromatase inhibitors, when used under medical supervision, can help manage hormonal imbalances and associated symptoms. It's crucial to approach hormone management with a comprehensive understanding of individual health needs and regular monitoring.
Artichokes |
Arugula |
Black Tea |
Blueberries |
Broccoli |
Brussel Sprouts |
Cabbage |
Cauliflower |
Celery |
Cherries |
Chives |
Cilantro |
Collard Greens |
Corn |
Cranberries |
Ligonberries |
Currants |
Bilberries |
Grapes |
Green Onions |
Green Tea |
Honey (Raw) |
Horseradish |
Peppers |
Lemons & Limes |
Mexican Oregano |
Mushrooms |
Mustard |
Mustard Greens |
Oats |
Oranges |
Parsley |
Passion Fruit |
Pomegranates |
Radishes |
Saffron |
Turnips |
Turnip Greens |
Walnuts |
Watercress |
Exercise
1. Resistance Training: Regular engagement in resistance or strength training exercises holds a pivotal role in sustaining hormonal equilibrium. Compound movements like squats, deadlifts, and weightlifting emerge as catalysts for increased testosterone production. By activating large muscle groups, such as incorporating lower-body exercises like squats and lunges alongside upper-body workouts, trigger a substantial hormonal response, elevating testosterone levels.
Heavy lifting, especially with full-body exercises like squats, deadlifts, and bench presses, is crucial for boosting testosterone. Use weights at 85-95% of your one-rep max (1RM) and aim for 2-3 full-body workouts per week. Beginners can start with weight machines before transitioning to free weights.
Longer rest periods (around 120 seconds) between sets are better for testosterone production. To make the most of your time, alternate between exercises that don't stress the same muscles. For example, pair bench presses with squats, taking shorter breaks between each.
Forced reps involve performing as many reps as possible, then having a partner assist with a few additional reps. This method has been shown to increase testosterone more effectively than solo reps. Incorporate forced reps into the last set of your exercises.
2. High-Intensity Interval Training (HIIT): HIIT workouts introduce brief, intense bursts of exercise followed by rest or lower-intensity periods. Integrating HIIT into your routine exhibits positive effects on testosterone levels. The dynamic nature of HIIT prompts the body's adaptive response, nurturing hormonal balance. Studies show that short, intense sprints can significantly boost testosterone levels. For optimal results, perform 5-10 sprints lasting no more than 15-30 seconds each, with full recovery between sprints (typically 3-4 times the sprint duration). Aim to do sprint workouts 2-3 times a week.
3. Cardiovascular Exercise: While resistance training takes center stage, cardiovascular exercise contributes significantly to overall health. Moderate-intensity cardio activities like jogging, cycling, jumping rope, rebounding, or sauna enhance cardiovascular well-being, complementing the body's holistic fitness.
4. Avoid Overtraining: Guarding against overtraining, characterized by excessive exercise without adequate recovery, is crucial for hormonal health. Prolonged intense workouts may elevate cortisol levels, a stress hormone with adverse effects on testosterone production. Adequate recovery time is essential to prevent the pitfalls of overtraining. Checking biometrics such as HRV is a great evidence-based indicator to quantify stress in the system.
A sample full-body workout three times a week might include:
- Warm-up
- 4 sets of 8 reps of bench press and squats
- 4 sets of 8 reps of deadlifts and pull-ups
- 6 sets of 10-second sprints
- Cool-down
A well-rounded exercise routine embraces resistance training (consisting of novel exercises to address specific musculoskeletal, biomechanical imbalances, and breathing mechanics), HIIT, and moderate-intensity cardio. Each type of exercise brings unique contributions to hormonal balance, offering comprehensive benefits for overall health. The body intricately adapts hormonally to the demands imposed during exercise. Thoughtfully designed, regular exercise routines can instigate positive hormonal adaptations, fostering improved testosterone regulation.
Acknowledging the diversity of individual responses to exercise is paramount. Tailoring routines to personal preferences and fitness levels ensures sustainable and enjoyable exercise habits, promoting long-term commitment. Consistency emerges as the linchpin for reaping long-term hormonal benefits from exercise. Establishing a regular routine that integrates various exercise types contributes not only to hormonal well-being but also to overall health.
In conclusion, the synergy of cardiovascular and resistance training exercises presents a potent strategy for optimizing testosterone levels. Resistance training, with a focus on compound movements and weightlifting, sparks testosterone production, while the inclusion of HIIT and cardiovascular exercise contributes holistically to health. Vigilance against overtraining and allowing sufficient recovery time are pivotal in maintaining hormonal balance. By adopting a balanced and personalized exercise routine, individuals can actively support hormonal health, enhancing their overall well-being.
Stress Management
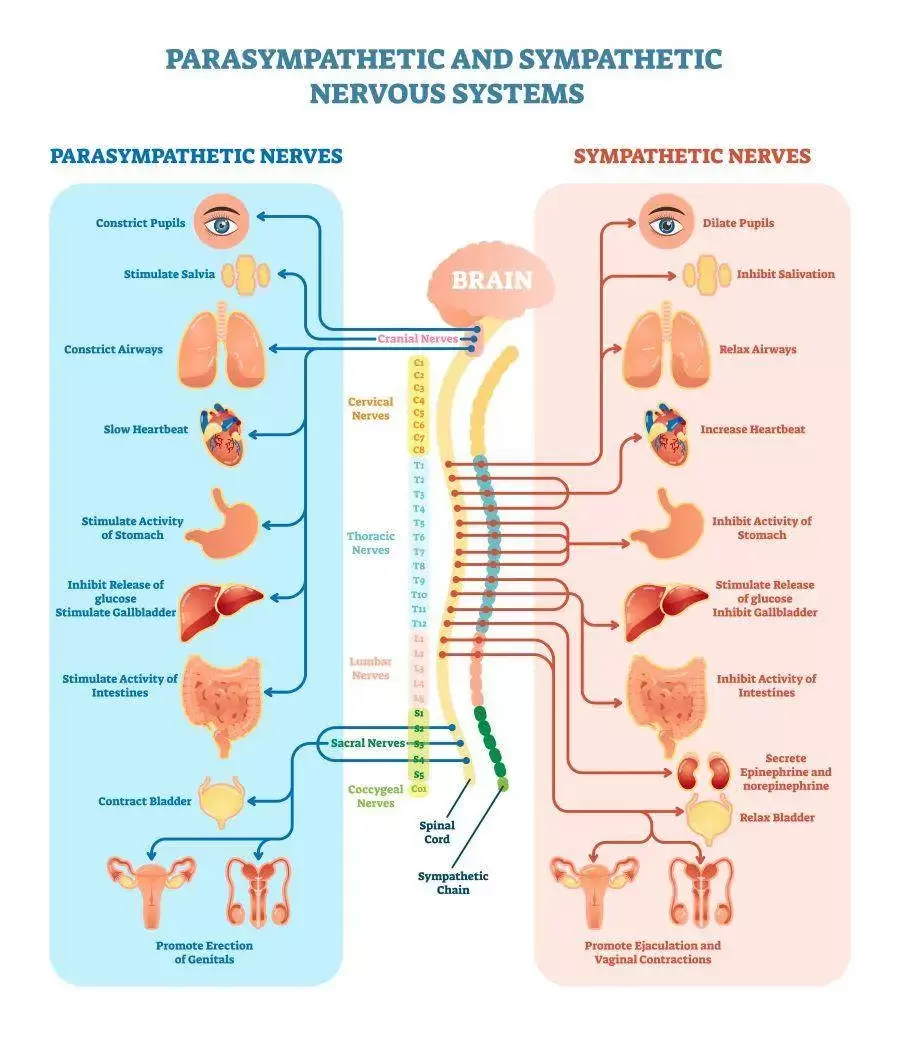
Techniques that induce relaxation activate the parasympathetic nervous system (PNS), which counters the fight-or-flight response associated with chronic stress. By calming the SNS, they help restore hormonal balance, positively impacting testosterone levels. This increase in the PNS can directly improve sleep quality. Quality sleep is essential for optimal hormonal function, including testosterone production during the night.
As with many of the solutions addressed, there is trend in the underlying mechanisms that result in improved hormonal balance, including reduced inflammation, improved mood and mental health, which fosters a mind-body connection that allows individuals to better understand and manage stress triggers. By increasing self-awareness, individuals can make conscious choices that positively impact their hormonal responses, thereby facilitating changes in thought patterns and behaviors related to stress. A more adaptive response to stressors can reduce the physiological impact on hormones, including testosterone.
Stress management practices positively influence the communication between the brain and endocrine glands. Improved hormonal communication supports optimal functioning of the hypothalamus, pituitary gland, and testes, essential for testosterone regulation.
As with most practices that induce positive health effects, regular practice offers cumulative benefits over time. Consistency in these techniques contributes to sustained stress resilience and supports ongoing hormonal health. Anyone interested in optimizing hormone levels is encouraged to adopt a holistic perspective of well-being by addressing physical, mental, and emotional aspects of health. A balanced and integrated approach to well-being positively influences hormonal health, including testosterone levels.
As mentioned, incorporating meditation, yoga, or mindfulness, music, dancing, humor and laughter - whatever helps you relax - into one's routine can be a powerful strategy for managing chronic stress and supporting hormonal health. It's essential to choose techniques that resonate with individual preferences and consistently practice them to reap the long-term benefits.

Cold Water Immersion
Cold exposure activates the hypothalamus, a region of the brain that plays a central role in the regulation of hormonal balance. The hypothalamus controls the release of gonadotropin-releasing hormone (GnRH), which, in turn, stimulates the pituitary gland to release luteinizing hormone (LH). LH acts on the testes, promoting the synthesis and release of testosterone.
From a vascular perspective, cold water immersion may lead to vasoconstriction (narrowing of blood vessels) followed by vasodilation (widening of blood vessels) in response to rewarming. This cycle of vasoconstriction and vasodilation can enhance blood flow, potentially increasing perfusion to the testes. Improved blood flow to the testes may support optimal Leydig cell function, which is crucial for testosterone production.
Metabolically, exposure to cold activates brown adipose tissue (BAT), a type of fat tissue that generates heat. BAT activation is associated with increased energy expenditure and metabolic activity. Some studies suggest that BAT activation may positively influence hormonal regulation, including testosterone production.
Cold water immersion has also been demonstrated to have anti-inflammatory effects. Chronic inflammation is associated with disruptions in not only energy production, but hormonal balance as well, including reduced testosterone levels. By reducing inflammation, cold water immersion may support a more favorable hormonal environment.
Additionally, some individuals report improved sleep quality following cold water immersion, likely due to enhanced thermoregulation. Quality sleep is crucial for overall health, including hormonal regulation. Improved sleep may indirectly contribute to optimal testosterone levels.
Cold exposure is considered a form of hormetic stress, stimulating cold shock proteins a mild stressor that, when applied in moderation, may lead to adaptive responses. Hormetic stressors, such as cold exposure, have been proposed to stimulate the body's adaptive mechanisms, including the endocrine system, potentially leading to increased testosterone production.
From a neurobiological perspective, cold water immersion has been associated with an increase in neurotransmitters, such as dopamine and norepinephrine levels, which can beneficially impact mood and behavior. However, what is not often described by proponents of cold water immersion is that exposure to extreme cold (determined by the individual's physiology, experience and perception) can trigger a cascade of physiological responses leading to elevated adrenaline (epinephrine) levels, thereby causing excess stress, and moving the needle in the opposite direction, away from optimal hormonal balance. Cold water immersion can certainly activate the sympathetic nervous system, otherwise known as the body's "fight or flight" response, which is why it is best to perform cold water immersion sometime in the morning or mid-day.
Dopamine and norepinephrine are neurotransmitters that play key roles in the regulation of mood, attention, and arousal. Cold water immersion has been shown to stimulate the release of both dopamine and norepinephrine in response to the stress of cold exposure. Dopamine has been suggested to have a regulatory role in the release of adrenaline. Studies indicate that dopamine, acting through specific receptors, may influence the release of adrenaline from the adrenal medulla. The overall response to cold stress involves the activation of the hypothalamus-pituitary-adrenal (HPA) axis, leading to the release of stress hormones, including cortisol and adrenaline.
It is important to realize that just because some is good, more is not always better. Deliberate cold exposure can absolutely improve quality of life, via hormonal mechanisms. However, if performed improperly, cold water immersion can disrupt hormonal effects. It is ideal to start low and slow. In other words, use a low dose and progressively increase as tolerance improves. As always, listen to your body. Shivering is a sign that the body is too cold.
Regulating Ejaculation Frequency
Recommended Ejaculation Frequency by Age:
- 20s: As desired
- 30s: 3-4 times per week
- 40s: 2-3 times per week
- 50s: 1-2 times per week
- 60+: Once a week, depending on health
A popular method among Tao practitioners is to ejaculate only two or three times out of every ten sexual encounters. Sun Simiao, a notable Tao theorist, advises men over 50 to ejaculate no more than once every 20 days, and men over 60 no more than once every 100 days.
To practice "injaculation" rather than ejaculation, men can squeeze the muscles used to stop urine flow while "breathing the energy" up the spine. If ejaculation seems imminent, applying pressure to the perineum (the area between the scrotum and anus) can help delay it. Elaboration of this technique can be found in the book, The Multiorgasmic Man.
This technique is cost-free but may require some patience to avoid becoming moody or aggressive due to sexual frustration. On the other hand, researchers at Boston University School of Public Health found that ejaculating at least 21 times a month may reduce the risk of prostate cancer.
Other Lifestyle Strategies
- Adequate Sleep: Ensure sufficient and quality sleep. Sleep plays a crucial role in hormonal regulation, including testosterone production. Aim for 7-9 hours of sleep per night. This also include mitigating non-native EMF exposure via blue lights in the environment. Blue light exposure is well documented to disrupt circadian rhythms, thereby impairing recovery mechanisms, and disrupting the delicate balance of hormones.
- Maintain a Healthy Weight: Achieve and maintain a healthy weight. Obesity is associated with lower testosterone levels, and losing excess weight can positively impact hormonal balance. A general rule of thumb is a healthy BMI, although for individuals who have large amounts of muscle, the reliability of BMI falls short.
- Vitamin D: Ensure adequate vitamin D levels. Vitamin D deficiency has been linked to lower testosterone levels, among many other comorbidities and increased all-cause mortality. It is ideal to spend time in sunlight at solar noon, and avoid vitamin D supplements. Our ancient ancestors spent nearly all of their time outside, and did not have access to synthetic products.
- Limit Alcohol Consumption: Moderate alcohol intake, as excessive alcohol consumption has been associated with lower testosterone levels. To date, there are zero benefits of alcohol consumption, perhaps (although loosely) with exception to social connection - of course, there are limits to the risk:benefit ratio with respect to the amount of alcohol consumed. Alcohol is a known toxin directly connected to a variety of comorbidities.
Navigating andropause involves a multifaceted approach that addresses both hormonal changes and lifestyle factors. By incorporating these science-backed nutrition and lifestyle strategies, men can optimize their well-being during this natural phase of life. Always consult with a healthcare professional for personalized advice based on individual health needs.
Optimizing Your Testosterone: A Day in the Life
Start your day with getting sun into your eyes. Afterwards, consume a breakfast rich in healthy fats like avocado, eggs, grass-fed butter and cheese, oyster mushrooms, sauerkraut, and coconut milk. Along with breakfast, take the following supplements:
- 5g creatine
- 50mg supplemental DHEA
- 10mg boron
- 30g cocoa powder
- 2g maca root extract
- 250 mg shilajit
- 500mg fenugreek extract
- 200mg Pycnogenol
- 200-300mg Eurycoma longifolia
- 300mg Tribulus
Throughout the Day:
Be sure to get sun on your skin at solar noon in an effort to create vitamin D. Implement EMF mitigation strategies by keeping your phone in airplane mode when not in use, avoiding using your laptop on your lap (or using an anti-radiation devices, such as Aires Tech), and turning off Wi-Fi on devices when using ethernet. Detox your home and consider auditing your workspace for EMF with an acoustimeter or by hiring a building biologist. Manage stress to maintain a high testosterone ratio. Practice relaxation techniques like deep nasal and belly breathing, laugh, smile, get outdoors, and be mindful of stress mitigation strategies. Spend time with people, especially women, as their presence can boost testosterone levels. Avoid pornography as it can negatively impact hormonal balance.
Afternoon Workout:
For an effective testosterone-boosting workout, do the following exercises with heavy weights, ensuring good form. Perform 5 sets of 5 reps each:
- Bench Press
- Deadlift
- Front Squat
- Shoulder Press
- Clean
Evening:
Enhance carbon dioxide levels to augment the efficiency of oxygen transport and energy production.
Integral Wellness Program: All in one Approach
1. Evidence-Based & Holistic Approach:
- Rooted in evidence-based practices, the Integral Wellness Program prioritizes approaches backed by scientific research.
- This credible and reliable program adopts a holistic model, recognizing the interconnectedness of physical, mental, and emotional well-being.
- By addressing multiple dimensions of health, it aims to create a synergistic effect, optimizing the conditions for hormonal balance.
- The program unfolds in a step-by-step manner, offering clear protocols for implementation.
- This structured approach simplifies the journey, making it accessible for individuals seeking a systematic and manageable process.
- Recognizing that each individual is unique, the program provides personalized guidance tailored to specific needs and goals.
- Customization ensures that the approach resonates with individual preferences and aligns with their health objectives.
- Integral to the program are nutritional strategies designed to support hormone optimization.
- These strategies likely include guidance on nutrient-dense foods, dietary patterns, and specific nutrients beneficial for hormonal health.
- Beyond nutrition, the program delves into lifestyle optimization.
- Factors such as sleep, stress management, and physical activity are likely addressed, recognizing their significant impact on hormonal balance.
- This holistic approach acknowledges the influence of mental well-being on hormonal health.
- The program likely provides educational resources, empowering individuals with knowledge about testosterone, hormonal health, and the impact of lifestyle choices.
- Informed decisions are pivotal to sustained well-being.
- The program is available through an accessible online platform, enabling participants to engage at their own pace and convenience.
- Flexibility in participation facilitates seamless integration into daily life.
In essence, the Integral Wellness Program serves as a comprehensive guide for those embarking on a journey to optimize testosterone levels. Through its holistic and step-by-step approach, individuals can navigate the intricacies of well-being, unlocking the potential for sustained hormonal health.
Movement | nutrition | lifestyle |
References
Dean, W. J., & Depledge, M. H. (2005). Stress and age-related changes in the male reproductive system. Toxicology and Industrial Health, 21(9-10), 247-255. [DOI: 10.1177/0748233705058995]
https://www.sciencedirect.com/science/article/abs/pii/S0741832900001245
Maggio, M., Lauretani, F., Ceda, G. P., Bandinelli, S., Ling, S. M., Metter, E. J., ... & Ferrucci, L. (2007). Association between hormones and metabolic syndrome in older Italian men. The Journal of the Federation of American Societies for Experimental Biology, 21(5), 1359-1366. [DOI: 10.1096/fj.06-7470com]
Epel, E., Jimenez, S., Brownell, K., Stroud, L., & Stoney, C. (2004). Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosomatic Medicine, 66(6), 967-974. [DOI: 10.1097/01.psy.0000141862.25270.64]
Hayes, L. D., Herbert, P., Sculthorpe, N., & Grace, F. M. (2015). Exercise training improves free testosterone in lifelong sedentary aging men. Endocrine Connections, 4(3), 152-162. [DOI: 10.1530/EC-15-0056]
Wang, C., Cunningham, G., Dobs, A., Iranmanesh, A., Matsumoto, A. M., Snyder, P. J., ... & Swerdloff, R. S. (2004). Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. The Journal of Clinical Endocrinology & Metabolism, 89(5), 2085-2098. [DOI: 10.1210/jc.2003-032006]
Zarrouf, F. A., Artz, S., Griffith, J., Sirbu, C., & Kommor, M. (2009). Testosterone and depression: systematic review and meta-analysis. Journal of Psychiatric Practice, 15(4), 289-305. [DOI: 10.1097/01.pra.0000358315.88931.fc]
Kenny, A. M., Prestwood, K. M., Gruman, C. A., Marcello, K. M., & Raisz, L. G. (2001). Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 56(5), M266-M272. [DOI: 10.1093/gerona/56.5.M266]
Corona, G., Rastrelli, G., Monami, M., Guay, A., Buvat, J., Sforza, A., ... & Maggi, M. (2011). Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. European Journal of Endocrinology, 165(5), 687-701. [DOI: 10.1530/EJE-11-0447]
Araujo AB, O'Donnell AB, Brambilla DJ, Simpson WB, Longcope C, Matsumoto AM, McKinlay JB. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2004 Dec;89(12):5920-6. doi: 10.1210/jc.2003-031719. PMID: 15579737.
Pitteloud N, Hardin M, Dwyer AA, Valassi E, Yialamas M, Elahi D, Hayes FJ. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metab. 2005 May;90(5):2636-41. doi: 10.1210/jc.2004-2190. Epub 2005 Feb 15. PMID: 15713702.
Mazur, A., & Booth, A. (1998). Testosterone and dominance in men.
Krassas, G. E., Pontikides, N., & Kaltsas, T. (2005). Thyroid function and androgens: A review.
Veldhuis, J. D., & Iranmanesh, A. (2009). Physiological regulation of the human growth hormone (GH)-insulin-like growth factor type I (IGF-I) axis: Predominant impact of age, obesity, gonadal function, and sleep.
Kocoska-Maras, L., & Comasco, E. (2019). Impact of sex hormones and gender on the course of bipolar disorder.
Becker, J. B. (2009). Sex differences in the neural mechanisms mediating addiction: A new synthesis and hypothesis.
Caruso, D., Pesaresi, M., Abbiati, F., Calabrese, D., Giatti, S., Garcia-Segura, L. M., & Melcangi, R. C. (2011). Comparison of plasma and cerebrospinal fluid levels of neuroactive steroids with their brain, spinal cord and peripheral nerve levels in male and female rats.
Brambilla, F., Savoia, P., Baschieri, F., Mancini, M., Petrillo, P., Montalbetti, L., & Santoro, A. (2003). Plasma concentrations of amino acids in patients with major depression and in normal controls.
Vermeulen, A., Kaufman, J. M., & Giagulli, V. A. (1996). Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. Journal of Clinical Investigation, 81(6), 1821-1826.
Health A-Z. (n.d.). Andropause.
Davidson, J. M., Camargo, C. A., & Smith, E. R. (1999). Effects of androgen on sexual behavior in hypogonadal men. Aging, Neuropsychology, and Cognition, 6(2), 87-103.
Harman, S. M., Metter, E. J., Tobin, J. D., Pearson, J., & Blackman, M. R. (2002). Longitudinal effects of aging on serum total and free testosterone levels in healthy men. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 57(2), M76-M83.
Jacobs, H. I. L., Priovoulos, N., Poser, B. A., Pagen, L. H. G., Ivanov, D., Verhey, F. R. J., ... & Dijkstra, A. A. (2023). Serotonin synthesis capacity in the dorsal raphe nucleus predicts aging-related hippocampal atrophy. Molecular Psychiatry. doi:10.1038/s41380-023-02177-x
Veldhuis, J. D., & Iranmanesh, A. (2009). Physiological regulation of the human growth hormone (GH)-insulin-like growth factor type I (IGF-I) axis: Predominant impact of age, obesity, gonadal function, and sleep. Journal of Endocrine Society, 3(1), 91-98. [DOI: 10.1210/jendso/bvz032]
Effect of testosterone on muscle mass and muscle protein synthesis
R. C. Griggs, W. Kingston, R. F. Jozefowicz, B. E. Herr, G. Forbes, and D. Halliday
Journal of Applied Physiology 1989 66:1, 498-503
Nelly Pitteloud, Vamsi K. Mootha, Andrew A. Dwyer, Megan Hardin, Hang Lee, Karl-Fredrik Eriksson, Devjit Tripathy, Maria Yialamas, Leif Groop, Dariush Elahi, Frances J. Hayes; Relationship Between Testosterone Levels, Insulin Sensitivity, and Mitochondrial Function in Men. Diabetes Care 1 July 2005; 28 (7): 1636–1642. https://doi.org/10.2337/diacare.28.7.1636
Uzunlulu, Mehmet, et al. “Association between Metabolic Syndrome and Cancer.” Annals of Nutrition and Metabolism, vol. 68, no. 3, 2016, pp. 173–179, www.karger.com/Article/FullText/443743, https://doi.org/10.1159/000443743.
Santen, R J. “Is Aromatization of Testosterone to Estradiol Required for Inhibition of Luteinizing Hormone Secretion in Men?” Journal of Clinical Investigation, vol. 56, no. 6, 1 Dec. 1975, pp. 1555–1563, https://doi.org/10.1172/jci108237. Accessed 22 Oct. 2020.
Carskadon, Mary A., et al. “Sleep Fragmentation in the Elderly: Relationship to Daytime Sleep Tendency.” Neurobiology of Aging, vol. 3, no. 4, Dec. 1982, pp. 321–327, https://doi.org/10.1016/0197-4580(82)90020-3.
Liu, Peter Y., and Radha T. Reddy. “Sleep, Testosterone and Cortisol Balance, and Ageing Men.” Reviews in Endocrine & Metabolic Disorders, vol. 23, no. 6, 1 Dec. 2022, pp. 1323–1339, pubmed.ncbi.nlm.nih.gov/36152143/, https://doi.org/10.1007/s11154-022-09755-4. Accessed 27 Jan. 2023.
Rosner W, Vesper H; Endocrine Society, et al. Toward excellence in testosterone testing: A consensus statement. J Clin Edocrinol Metab. 2010 Oct; 95(10):4542-4548. Pubmed 20926540
Szumilas, Kamila, et al. “The Effects of E-Cigarette Vapor Components on the Morphology and Function of the Male and Female Reproductive Systems: A Systematic Review.” International Journal of Environmental Research and Public Health, vol. 17, no. 17, 24 Aug. 2020, p. 6152, https://doi.org/10.3390/ijerph17176152.
Zhang X, Xiao J, Liu Q, Ye Y, Guo W, Cui J, He Q, Feng W, Liu M. Low Serum Total Testosterone Is Associated with Non-Alcoholic Fatty Liver Disease in Men but Not in Women with Type 2 Diabetes Mellitus. Int J Endocrinol. 2022 Aug 27;2022:8509204. doi: 10.1155/2022/8509204. PMID: 36065220; PMCID: PMC9440833.
Van der Burgh AC, Khan SR, Neggers SJCMM, Hoorn EJ, Chaker L. The role of serum testosterone and dehydroepiandrosterone sulfate in kidney function and clinical outcomes in chronic kidney disease: a systematic review and meta-analysis. Endocr Connect. 2022 Jun 23;11(6):e220061. doi: 10.1530/EC-22-0061. PMID: 35551117; PMCID: PMC9254301.
Josephs, Robert A., et al. “Dual-Hormone Stress Reactivity Predicts Downstream War-Zone Stress-Evoked PTSD.” Psychoneuroendocrinology, vol. 78, Apr. 2017, pp. 76–84, https://doi.org/10.1016/j.psyneuen.2017.01.013. Accessed 19 Feb. 2022.
Haren, M. T., Siddiqui, A. M., Armbrecht, H. J., Kevorkian, R. T., Kim, M. J., Haas, M. J., ... & Orwoll, E. S. (2007). Testosterone modulates gene expression pathways regulating nutrient accumulation, glucose metabolism and protein turnover in mouse skeletal muscle. https://doi.org/10.1093/humrep/dew234
Kaplan, S. A., Meehan, A. G., Shah, A., & Meller, J. (2007). Patterns of hypogonadism and hormone changes in hypogonadal men following long-term clomiphene citrate use for atypical hypogonadism. https://doi.org/10.1210/jc.2006-1853
Moskovic, D. J., Arafa, M., & Lipshultz, L. I. (2017). Klinefelter syndrome and its variants: an update and review for the primary care physician. https://doi.org/10.1093/humupd/dmx022
Rohrmann, S., Nelson, W. G., Rifai, N., Brown, T. R., Dobs, A., Kanarek, N., ... & Platz, E. A. (2007). Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. https://pubmed.ncbi.nlm.nih.gov/17635834/
Wang, C., Catlin, D. H., Starcevic, B., Heber, D., Ambler, C., Berman, N., ... & Swerdloff, R. S. (2004). Low-fat high-fiber diet decreased serum and urine androgens in men. https://doi.org/10.1093/jn/134.8.1889
Oh, T. H., Kim, K. W., Kim, J. H., Cho, H. J., Shin, J. H., Kim, T. H., ... & Kim, T. H. (2020). Relationship Between Serum Cortisol Level and Myasthenia Gravis: A Case–Control Study. https://doi.org/10.1002/mus.27039
Gunnars, K. (2022). 11 Natural Ways to Lower Your Cortisol Levels. Healthline. https://www.healthline.com/nutrition/ways-to-lower-cortisol
Pournaras, D. J., le Roux, C. W., & Macdonald, I. A. (2015). The effect of bariatric surgery on intestinal absorption and transit time. https://doi.org/10.1016/j.cmet.2015.01.004
Glassman, K. (2018). Sugar Is Wreaking Havoc on Your Hormonal Health. Observer. https://observer.com/2018/02/sugar-is-wreaking-havoc-on-your-hormonal-health/
Norton, B. (2016). Fat Doesn't Make You Fat. InBody USA. https://inbodyusa.com/blogs/inbodyblog/90571521-fat-doesnt-make-you-fat/
Lumen Learning. (n.d.). Lipids. https://courses.lumenlearning.com/microbiology/chapter/lipids/
Norton, B. (2016). Fat Doesn't Make You Fat. InBody USA. https://inbodyusa.com/blogs/inbodyblog/90571521-fat-doesnt-make-you-fat/
Lear, S. A., James, P. T., Ko GT, et al. (2009). Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. https://doi.org/10.1016/j.jclinepi.2009.07.008
Hernández-Díaz, S., Schisterman, E. F., Hernán, M. A., & The Birth weight modifies the association between maternal age and type 1 diabetes: a population-based cohort study. https://doi.org/10.1186/1741-7015-5-5
Gunnars, K. (2021). The 6 Main Ways to Reduce Trans Fat Intake. Healthline. https://www.healthline.com/nutrition/trans-fat-foods
Brughelli, M., Cronin, J., & Chaouachi, A. (2008). Effects of running velocity on running kinetics and kinematics. https://doi.org/10.1007/s40279-013-0051-4
Nordic Naturals. (n.d.). Omega-3 Essential Fatty Acids. https://www.nordicnaturals.com/healthy-science/omega-3-essential-fatty-acids/
Glick-Bauer, M., & Yeh, M. C. (2014). The health advantage of a vegan diet: exploring the gut microbiota connection. https://doi.org/10.3389/fnut.2014.00024
Li, K., Huang, T., Zheng, J., Wu, K., & Li, D. (2016). Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor α: a meta-analysis. https://doi.org/10.1038/ejcn.2015.255
Jouris, K. B., McDaniel, J. L., & Weiss, E. P. (2010). The effect of omega-3 fatty acid supplementation on the inflammatory response to eccentric strength exercise. https://doi.org/10.1186/1550-2783-7-31
Fukuoka, M. (2008, November 13). Chemical-Based Farming Systems Robbing Us of Nutrients. Permaculture News. https://www.permaculturenews.org/2008/11/13/chemical-based-farming-systems-robbing-us-of-nutrients/
Yamada, Y., Kamura, T., Sato, A., & Watanabe, T. (2004). Changes in soil chemical properties of paddy fields under conventional and no-tillage cultivation. https://www.ars.usda.gov/ARSUserFiles/50701000/cswq-0426-yamada.pdf
Druker, S. (2010). Scientists Find Negative Impacts of GM Crops. The Non-GMO Report. https://nongmoreport.com/articles/jan10/scientists_find_negative_impacts_of_GM_crops.php
Schor, Jacob. “Emotions and Health: Laughter Really Is Good Medicine.” Www.naturalmedicinejournal.com, 14 Jan. 2014, www.naturalmedicinejournal.com/journal/emotions-and-health-laughter-really-good-medicine.
Yeap, Bu B, et al. “Associations of Testosterone and Related Hormones with All-Cause and Cardiovascular Mortality and Incident Cardiovascular Disease in Men.” Annals of Internal Medicine, 14 May 2024, https://doi.org/10.7326/m23-2781. Accessed 28 May 2024.
Okechukwu, Chidiebere Emmanuel. “Does the Use of Mobile Phone Affect Male Fertility? A Mini-Review.” Journal of Human Reproductive Sciences, vol. 13, no. 3, 2020, p. 174, https://doi.org/10.4103/jhrs.jhrs_126_19.
Greenfield, Ben. “Increase Testosterone Levels Naturally: Biohacking Men’s Health.” Ben Greenfield Life - Health, Diet, Fitness, Family & Faith, 23 Apr. 2024, bengreenfieldlife.com/article/hormones-articles/the-best-ways-to-increase-testosterone/?utm_source=Klaviyo&utm_medium=campaign&utm_campaign=testosterone%20article%204%2F23.
Leave a Reply.
Archives
May 2024
April 2024
March 2024
February 2024
January 2024
December 2023
September 2023
August 2023
June 2023
October 2022
July 2022
February 2022
January 2022
December 2021
November 2021
October 2021
August 2021
July 2021
June 2021
March 2021
February 2021
January 2021
December 2020
November 2020
September 2020
August 2020
March 2020
December 2019
November 2019
October 2019
September 2019
August 2019
July 2019
May 2019
April 2019
March 2019
February 2019
January 2019
December 2018
November 2018
October 2018
September 2018
August 2018
July 2018
June 2018
April 2018
March 2018
February 2018
January 2018
December 2017
November 2017
October 2017
September 2017
August 2017
July 2017
June 2017
May 2017
April 2017
March 2017
February 2017
January 2017
Categories
All
5G
Adductors
Anxiety
Autism
Ayurveda
Big Pharma
Body
Breathing
Cancer
Cannabis
Carbohydrates
Cardiovascular Disease
Children
Chronic Disease
Cognition
Consciousness
Coronavirus
Covid
Cryotherapy
Depression
Deuterium
Diet
Dietary Guidelines
EMFs
Emotions
Endocrine Disruptors
Environment
Exercise
Farming
Fasting
Fats
Fluoride
Food
Food Like Product
Food-like Product
Forest
Gardening
Genetics
Glyphosate
GMOs
Hamstrings
Healing
Health
Herbalism
Hormones
HRV
Immunity
Infertility
Laughter
Lockdowns
Loneliness
Longevity
Masks
Meditation
Metabolism
Microbiome
Microwave Radiation
Mind
Mortality
Musculoskeletal System
Nature
Neuroplasticity
Nutrition
Omega 3
Omega-3
Organic
Pelvis/Thigh
Pesticides
Physical Therapy
Placebo
Pollution
Positive
Pregnancy
Prevention
Processed Foods
Psi
Quadriceps
Research
Retirement
Salt
Sleep
Spine/Thorax
Spirit
Stress
Sugar
Technology
Touch Screens
Toxicity
Vaccines
Well Being
Well-Being







 RSS Feed
RSS Feed

