|
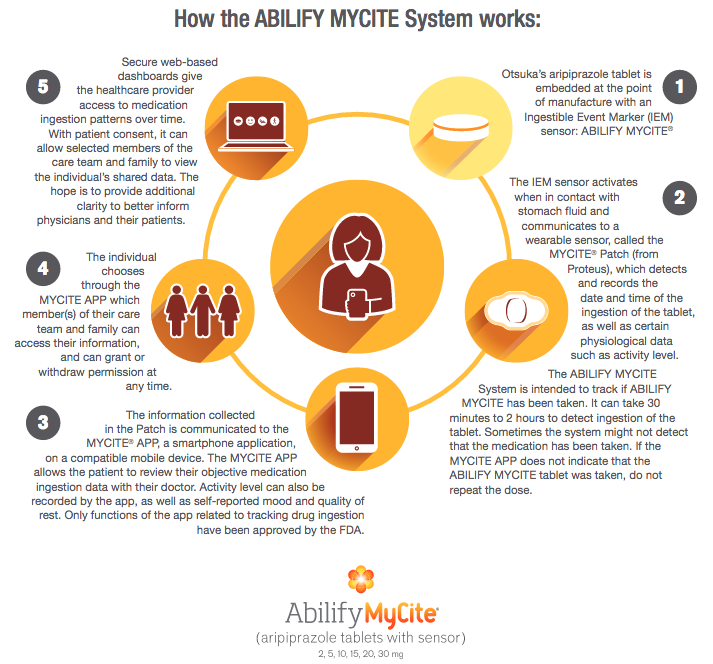
The Food and Drug Administration has approved the first digital pill — a medication embedded with an ingestible sensor the size of a grain of sand that can alert doctors whether, and when, patients take their medicine. The approval — announced on Monday, November 13, 2017 — marks a significant advance in the growing field of digital devices designed to monitor medicine-taking and to address the expensive, longstanding problem that millions of patients do not take drugs as prescribed (Iuga, & McGuire, 2014). It is estimated that nonadherence or noncompliance to medication costs about $100 billion a year, much of it because patients get sicker and need additional treatment or hospitalization (Viswanathan et al., 2012). Abilify, a antipsychotic medication, was originally approved by the FDA in 2002 for the treatment of schizophrenia, bipolar disorder, and in conjunction with an antidepressant, major depressive disorder. Patients who agree to take Abilify MyCite (aripiprazole tablets with sensor), the digital version of the medication, must sign consent forms allowing their doctors and up to four other people, such as family members, to receive electronic data showing date and time stamps, and the dosage of pills ingested. The patient can revoke access at any time. The pill is fitted with a tiny sensor, containing copper, magnesium and silicon, that communicates with a patch worn by the patient — the patch then transmits medication data to a smartphone app in which the authorized persons are able to see. An electrical signal is activated when the sensor comes into contact with stomach acid — the sensor then passes through the body naturally. A patch the patient wears on their left rib cage receives the signal several minutes after the pill is ingested. The patch then sends data to a smartphone app over Bluetooth, and must be replaced every seven days. The app allows patients to add activity level, their mood and the hours they have rested, then transmits the information to a database that can be accessed by those who have permission. Although this digital pill has the potential to improve public health, especially for patients who want to take their medication but forget, if used improperly, it could foster more mistrust instead of trust. Although voluntary, the technology is still likely to prompt questions about privacy and whether patients might feel pressure to take medication in a form their doctors can monitor. While ethical for a fully competent patient, a digital drug sounds like a potentially coercive tool. Insurers might eventually give patients incentives to use them, like discounts on copayments, adding that ethical issues could arise if the technology was so much incentivized that it almost is like coercion. Another controversial use might be requiring digital medicine as a condition for parole or releasing patients committed to psychiatric facilities. The newly approved pill is a collaboration between Abilify’s manufacturer, Otsuka, and Proteus Digital Health, a California company that created the sensor. Otsuka has not determined a price for Abilify MyCite, which will be released next year, first to a limited number of health plans. The price, and whether digital pills improve adherence, will greatly affect how widely they are used. Dr. Jeffrey Lieberman, chairman of psychiatry at Columbia University and NewYork-Presbyterian Hospital, noted it has only been approved to track doses, and has not yet been shown to improve adherence. He added there is any data currently to say it will improve adherence, but that will likely be studied after sales begin. While embedding digital technology in medications does open up many intriguing treatment avenues, it does raise privacy concerns as well. Would you want an electrical signal coming out of your body strong enough so your doctor (and others) can read it? Who knows what else a sensor in a pill could eventually track and how this data may be used? References Belluck, P. (2017). First Digital Pill Approved to Worries About Biomedical 'Big Brother'. [online] Nytimes.com. Available at: https://www.nytimes.com/2017/11/13/health/digital-pill-fda.html [Accessed 16 Nov. 2017].
Fda.gov. (2017). FDA approves pill with sensor that digitally tracks if patients have ingested their medication. [online] Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm584933.htm [Accessed 16 Nov. 2017]. Iuga, A. O., & McGuire, M. J. (2014). Adherence and health care costs. Risk Management and Healthcare Policy, 7, 35–44. http://doi.org/10.2147/RMHP.S19801 Viswanathan, M., Golin, C., Jones, C., Ashok, M., Blalock, S., Wines, R., Coker-Schwimmer, E., Rosen, D., Sista, P. and Lohr, K. (2012). Interventions to Improve Adherence to Self-administered Medications for Chronic Diseases in the United States. Annals of Internal Medicine, [online] 157(11), p.785. Available at: http://dx.doi.org/10.7326/0003-4819-157-11-201212040-00538 [Accessed 16 Nov. 2017].
0 Comments
Leave a Reply. |
This feed contains research, news, information, observations, and ideas at the level of the world.
Archives
May 2024
Categories
All
|
 RSS Feed
RSS Feed

