|
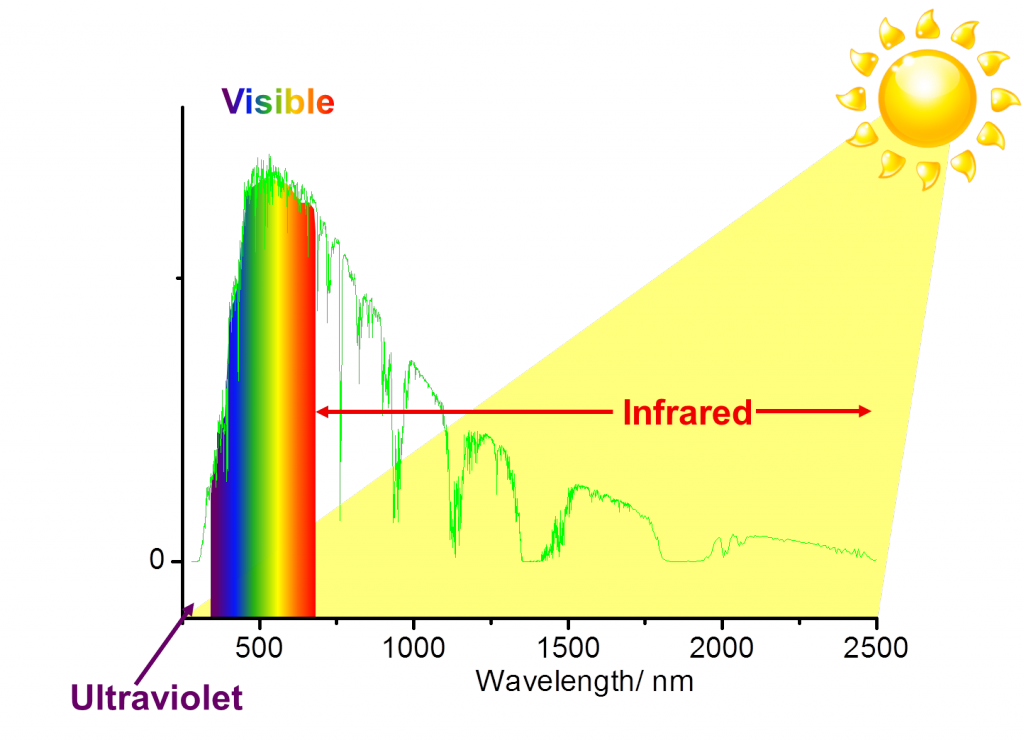
In the realm of emerging wellness technologies, red and near-infrared light therapy stands out as a beacon of potential health benefits. This non-invasive treatment, also known as photobiomodulation, has gained attention for its positive effects on various aspects of health, particularly in skin and muscle recovery. Let's delve into the illuminating world of red and near-infrared light therapy and explore how it can enhance your well-being. Photobiomodulation (PBM)Photobiomodulation, often referred to as PBM therapy, is a non-invasive and non-thermal treatment that utilizes light to stimulate various cellular processes. This therapeutic approach harnesses the power of specific wavelengths of light to enhance cellular function, promote tissue repair, and reduce inflammation. Among the key contributors to PBM are red and near-infrared (NIR) light. The Sun: the Original PhotobiomodulatorThe Sun emits energy in various forms, each associated with specific ranges of the electromagnetic spectrum. Here's a breakdown of the forms of energy emitted by the Sun: 1. Visible Light: This is the portion of solar radiation that is visible to the human eye, creating the spectrum of colors we perceive.
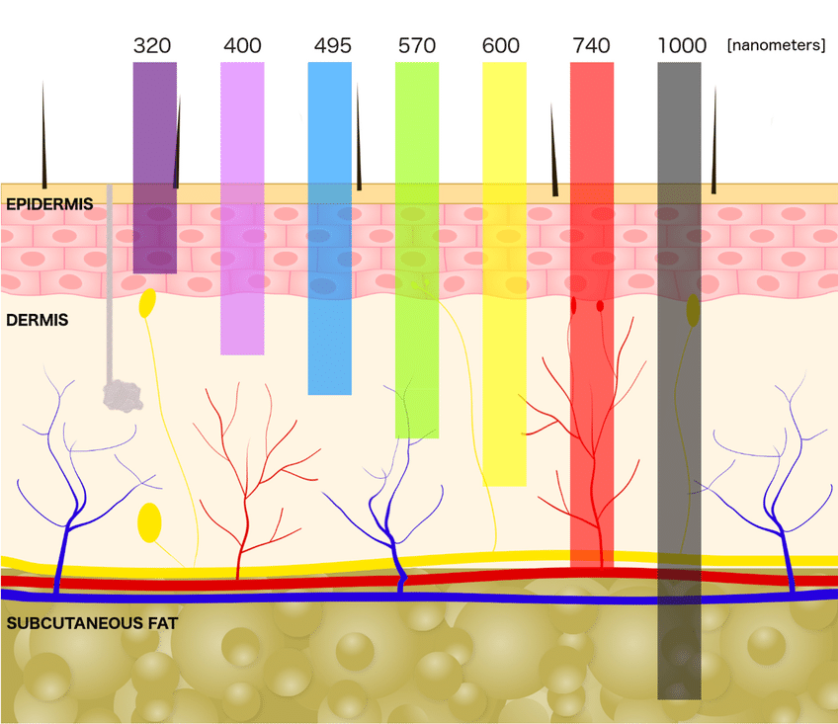
Red and Near-Infrared Light TherapyRed and near-infrared (NIR) light therapy involves exposing the body to light in the red (600-700 nanometers) and NIR (700-1100 nanometers) spectrums. These wavelengths are known to penetrate the skin and underlying tissues, interacting with cellular structures and triggering beneficial responses. Skin Layers and Light Penetration: The human skin consists of several layers, each with distinct properties and functions. Understanding how red and NIR light navigate through these layers provides insight into their therapeutic reach.
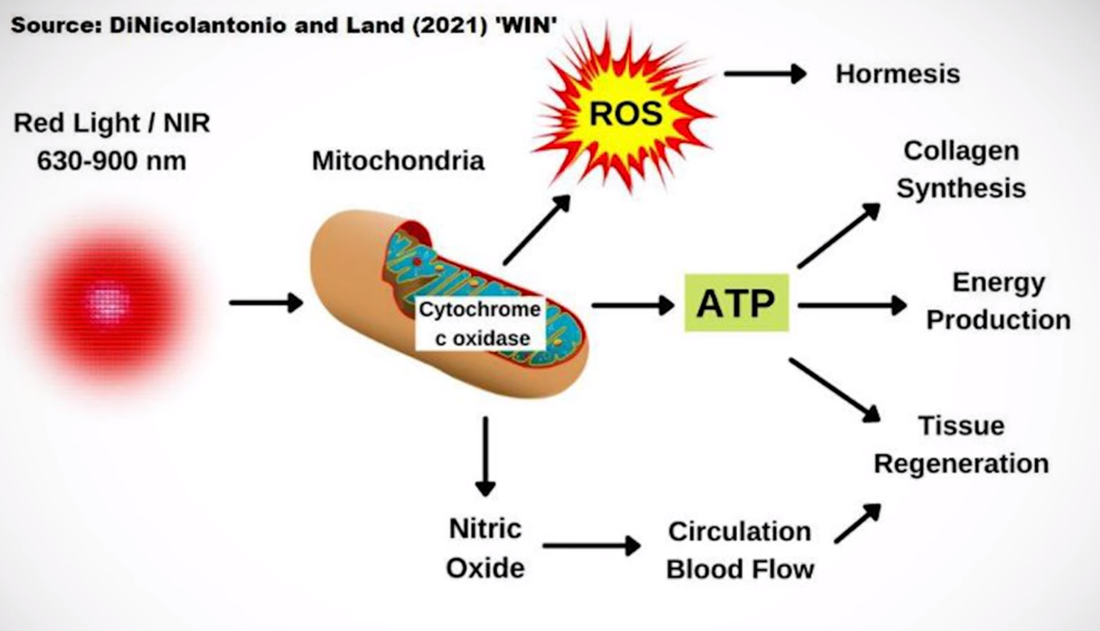
As red and NIR light journey through the skin layers, they engage with chromophores—molecules that absorb specific wavelengths of light. Key chromophores include cytochrome c oxidase, found in mitochondria, and various light-absorbing proteins and enzymes. These interactions trigger a cascade of cellular events, such as enhanced ATP production, increased circulation, and modulation of inflammatory responses. Understanding the depth of light penetration is crucial for tailoring red and NIR therapy to specific therapeutic goals. While red light may be more suitable for surface-level applications like skin rejuvenation, NIR light's ability to reach deeper tissues makes it a preferred choice for addressing musculoskeletal conditions and promoting systemic benefits. Mechanisms of Action
Influence of NIR on WaterNIR light can affect the structure of water molecules through a process known as photodissociation or photolysis. This process involves breaking molecular bonds using light energy. While NIR light does not directly ionize water molecules, it can influence their structure in several ways:
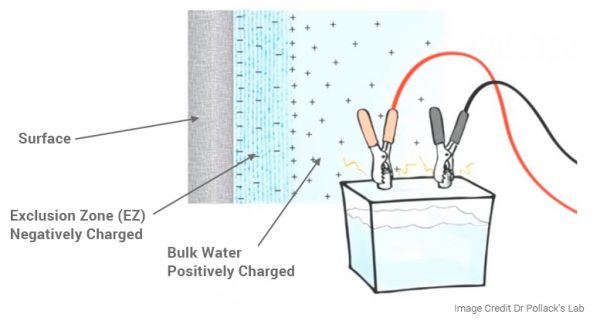
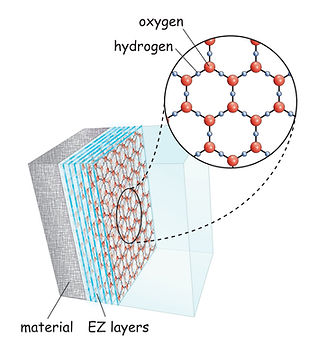
The concept of NIR light influencing the structuring of water into exclusion zone (EZ) water, also known as the fourth phase of water, is associated with the work of Dr. Gerald Pollack. According to Pollack's research, EZ water differs in its molecular arrangement from ordinary water, showing a structured pattern that extends beyond the traditional liquid structure. Formation of Exclusion Zone (EZ) Water:
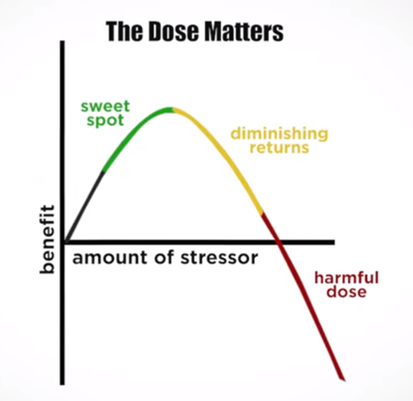
It's important to note that the exact mechanisms and effects of NIR light on water structure are complex and may vary depending on factors such as the wavelength of the light, intensity, and exposure duration. The idea of NIR light structuring water has gained attention in various fields, including alternative health practices, and environmental research. The field of water structuring and its potential implications for cellular biology is still an area of active investigation within scientific communities. Harnessing the Power of Hormetic Stress: The Biphased Dose Response of Red Light TherapyRed light therapy emerges not only as a therapeutic modality but as a potent hormetic stressor, operating on a biphasic dose-response principle. In the realm of hormesis, a phenomenon where exposure to low doses of stressors triggers adaptive responses, red light therapy takes center stage by demonstrating a nuanced and beneficial relationship with the body's stress response. Hormesis, in its essence, is the body's ability to adapt and respond positively to low doses of stress. Red light therapy, with its application of low levels of red and NIR light, acts as a hormetic stressor, initiating adaptive responses that strengthen cellular resilience and overall well-being. The biphasic dose response of red light therapy implies that its effects on the body follow a distinctive pattern. At lower doses, red light elicits a positive response, triggering cellular repair, mitochondrial enhancement, and anti-inflammatory effects. As the dose increases, the beneficial effects continue, but there's a point where diminishing returns occur. Understanding this biphasic response allows for optimized use of red light therapy for maximum benefit. Understanding the biphasic dose response of red light therapy allows for a personalized and optimal application. Tailoring the dose to individual needs ensures that the therapy remains within the hormetic zone, where the benefits are maximized without reaching a point of diminishing returns. Incorporating red light therapy into your wellness routine becomes a strategic choice for embracing hormetic stress. The biphasic dose response unfolds a journey of cellular resilience, mitochondrial optimization, and enhanced well-being, showcasing red light therapy as a dynamic and adaptive ally in the pursuit of holistic health. Applications: The Science Behind Red and Near-Infrared Light Therapy
Incorporating Light Therapy into Your Routine
Experience Revolutionary Healing with Boncharge Red Light Therapy DevicesUnlock the potential of cutting-edge healing with Boncharge Red Light Therapy devices, designed to redefine your wellness journey. These state-of-the-art devices boast a range of features aimed at maximizing therapeutic benefits while prioritizing your safety and comfort.
For as little as 10 minutes per day (ideally in the morning), you can incorporate Boncharge Red Light Therapy devices into your wellness routine and embark on a journey of transformative healing. Elevate your experience with the assurance of low EMF, flicker-free LEDs, convenient built-in timers, and the flexibility to customize your sessions. Boncharge is not just a device; it's a commitment to evidence-based wellness, empowering you to thrive with the power of red light therapy. Safety and ConsiderationsRed and NIR light therapy is generally considered safe with minimal side effects. However, appropriate device selection, treatment duration, and wavelength specificity are crucial for optimizing therapeutic outcomes. It is very important to do a photosensitivity test prior to long sessions with your red light therapy device. To perform the test, turn on both the red and NIR functions on your red light therapy device and shine the light on an exposed part of your forearm, about 3 inches to 1 foot away, for 4 minutes. If you experience any discomfort or redness on your skin it is encouraged to not use your red light therapy device until you have sought professional advice from your doctor or healthcare provider. Illuminating cellular healingIn conclusion, red and NIR light therapy, within the realm of PBM, represents a promising avenue for promoting cellular healing and overall well-being. As research continues, the applications and benefits of this non-invasive therapy are expected to expand. Red and NIR light therapy presents a compelling avenue for enhancing skin health and muscle recovery. As this technology continues to be explored, its potential applications in various fields, from sports medicine to dermatology, are expanding. By harnessing the power of light, individuals can embark on a journey toward improved vitality, resilience, and overall well-being. As with any wellness practice, informed decisions and professional guidance contribute to a holistic approach to health. references
1 Comment
Whether baby wipes are toxic or not depends on the ingredients. Some popular brands, such as Huggies or Pampers, that are known for their baby scent use an astounding amount of chemicals to achieve that scent. These chemicals are toxic for both babies and the environment. Baby wipes using natural and organic ingredients typically do not contain these additional chemicals, making them wonderful non-toxic baby wipes. Common baby wipe ingredients to avoidMany moms are well-intended to select the common brands of baby wipes. Afterall, baby wipes are packed with chemicals to make them efficient, a little more durable than toilet paper, and smell beautiful. However, some of those chemicals are not good for your baby. It is in the best interest of the consumer, the mom and baby, to make sure to read the package before you purchase a pack of disposable baby wipes. Here are some ingredients to look out for: fragranceThe fragrance is a general term that is used to encompass several different ingredients. If an ingredient contributes to making the wipes smell good, it can be labeled as “fragrance.” Many of these fragrances are petro-chemicals and are therefore toxic, and some of them are just not good for your little one’s skin. Instead, opt for fragrance-free wipes. Babies naturally smell adorable! If you do wind up buying baby wipes that are scented, double-check the ingredient list. Companies can use the term fragrance to encompass harmful ingredients. They are not required to list the ingredients or chemicals that are used to make their baby wipes smell the way that they do because this is considered a trade secret. This means that if the fragrance is created using parabens, for example, the company does not have to tell you because it is in the “fragrance” category. Fragrances have been linked to all sorts of health conditions, from autoimmune issues including contact dermatitis, and migraines and respiratory issues. FormaldehydeMake no mistake - you read that right. Formaldehyde has made headlines in recent years as it’s been found in baby wipes. This chemical is a known carcinogen, meaning that it can cause cancer. It’s also a known irritant causing allergic reactions in babies. Additionally, formaldehyde is known to be:
Researchers have observed over half of the popular baby wipe brands that were tested released formaldehyde during a study. What is even scarier is that none of the wipes listed formaldehyde as an ingredient. A current examination of the scientific data collected on the exposure to formaldehyde is associated with the following health conditions and abnormal physiologic events:
TriclosanThis antibacterial ingredient found in soaps and other products has been linked to allergies, endocrine disruption, weight gain and inflammatory responses, and may aggravate the growth of liver and kidney tumors. It’s also used commonly as a preservative. However, Triclosan was recently removed from antibacterial hand soaps because it can pass through the skin, which will lead to it having an effect on the body. It’s known to cause an allergic reaction as well as disrupt hormones. When triclosan is broken down, it can turn into Dioxin, which is known to cause cancer. Here is list of the known physiologic mechanisms in which triclosan exerts it's harmful effects:
Exposure to, and the consumption of triclosan is associated with the following health conditions and abnormal physiologic events:
Propylene GlycolPropylene glycol, also known as propane-1,2-diol, is compound used for various cosmetic, pharmaceutical and industrial applications, including as a solvent, humectant, preservative, and surfactant. Propylene glycol consumption results in kidney, liver, and neurologic toxicity, and it is certainly not recommended for pregnant women. Propylene glycol is a skin irritant, it may increase the absorption of other harmful excipients, and disrupts permeability of the blood brain barrier. When used in products like baby wipes, propylene glycol is intended to increase skin absorption of any ingredients more effectively, and at a quicker rate. This results in the body absorbing all of the toxic ingredients that are found in baby wipes, so it’s best to avoid this ingredient entirely. Polyethylene GlycolPolyethylene Glycol (PEG), otherwise known as Macrogol, Carbowax and many other derivatives when combined with other substances. PEG is a synthetic chemical compound derived from petroleum that is widely used for a variety of uses, including as a moisture carrier, solvent, preservative, thickener, and much more. PEG is another chemical that will help the skin absorb the ingredients of the baby wipes. Even though it’s a different chemical that is used, it still does the same thing as propylene glycol, which helps your little one absorb chemicals which may include carcinogens, including ethylene oxide, and potentially dioxane depending on the manufacturer. PEG is classified as biologically inert by our FDA. It is the “Gold Standard” for use in many medications to increase the blood clearance time, or in other words, the time it remains in one’s system, thereby enhancing drug effect. It is also used in drug manufacturing as an excipient for long term stabilization, bulking, and other therapeutic enhancements. It is used as a coating to prevent bacterial adhesion on orthopedic screws and sutures. In addition to medical uses, PEG is also used in cosmetics, foods, industrial applications, and other health and beauty products such as soaps, shampoos, toothpastes. It is also used as an e-cigarette liquid. PEG is everywhere in our environment, which is what many have surmised has led to a high percentage of the US population developing anti-PEG antibodies. This, of course, presents a significant challenge to those who rely on this substance in their manufacturing. Scientific studies to quantify the seriousness of the problem estimate that approximately 72% of the US population has acquired anti PEG antibodies. The referenced study used blood samples taken from 1990-1999 and earlier, showing a steady increase over time in the percentage of those with antibodies to PEG, making it conservative to estimate, after two decades, that the incidence is closer to 80% today. This circumstance has concerned the medical and pharmaceutical communities as an equally effective alternative has escaped identification, although several have been suggested, and because the great cost of shifting to such an alternative. Not only is PEG a “stealth” medicinal additive, delaying blood clearance due to its properties, but it is a stealth allergen, the vast majority of the population never having heard of it and many in the healthcare industry being unaware of its antigenic properties. A physician survey found: “Although 91% of respondents were aware of antidrug antibodies in general, only 22% were aware of APA (Anti-PEG Antibody) responses. Further, there was limited awareness (35%) of PEG’s inclusion in prescribed PEGylated therapeutics.” "Scientific studies to quantify the seriousness of the problem estimate that approximately 72% of the US population has acquired anti PEG antibodies." sodium benzoateSodium benzoate is also used as a preservative, to extend the shelf-life of products, and is actually the sodium salt of benzoic acid. The substance is an odorless, crystalline powder made by combining benzoic acid and sodium hydroxide, per a December 2015 article in Biotechnology and Health Sciences. Chemical exposure and consumption of sodium benzoate is linked with a variety of health conditions and abnormal physiologic events, including but not limited to:
safe Baby wipesTerra biodegradable wipes are durable and extra moist, with an easy dispensing flip-top lid. They are cross-woven for a soft cloth-like texture that is non-irritating while cleaning baby's delicate skin. Terra provides transparency with their ingredients:
referencesLiou, Yujie Linda, et al. “Formaldehyde Release from Baby Wipes: Analysis Using the Chromotropic Acid Method.” Dermatitis, vol. 30, no. 3, May 2019, pp. 207–212, https://doi.org/10.1097/der.0000000000000478.
Steinemann, Anne. “Health and Societal Effects from Exposure to Fragranced Consumer Products.” Preventive Medicine Reports, vol. 5, Mar. 2017, pp. 45–47, https://doi.org/10.1016/j.pmedr.2016.11.011. McCann, Donna, et al. “Food Additives and Hyperactive Behaviour in 3-Year-Old and 8/9-Year-Old Children in the Community: A Randomised, Double-Blinded, Placebo-Controlled Trial.” The Lancet, vol. 370, no. 9598, Nov. 2007, pp. 1560–1567, www.thelancet.com/journals/lancet/article/PIIS0140-6736(07)61306-3/fulltext, https://doi.org/10.1016/s0140-6736(07)61306-3. Nair, B. “Final Report on the Safety Assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate.” International Journal of Toxicology, vol. 20 Suppl 3, 2001, pp. 23–50, www.ncbi.nlm.nih.gov/pubmed/11766131, https://doi.org/10.1080/10915810152630729. Fujitani, T. “Short-Term Effect of Sodium Benzoate in F344 Rats and B6C3F1 Mice.” Toxicology Letters, vol. 69, no. 2, 1 Aug. 1993, pp. 171–179, pubmed.ncbi.nlm.nih.gov/8212059/, https://doi.org/10.1016/0378-4274(93)90102-4. Tsay, Huey-Jen, et al. “Treatment with Sodium Benzoate Leads to Malformation of Zebrafish Larvae.” Neurotoxicology and Teratology, vol. 29, no. 5, 1 Sept. 2007, pp. 562–569, pubmed.ncbi.nlm.nih.gov/17644306/, https://doi.org/10.1016/j.ntt.2007.05.001. Hu, Mingqian, et al. “[Analysis of Sodium Benzoate Biotoxicity by Atomic Force Microscope].” Sheng Wu Gong Cheng Xue Bao = Chinese Journal of Biotechnology, vol. 24, no. 8, 1 Aug. 2008, pp. 1428–1432, pubmed.ncbi.nlm.nih.gov/18998546/, https://doi.org/10.1016/s1872-2075(08)60064-3. Oyanagi, Kazuhiko, et al. “Cytotoxicities of Sodium Benzoate in Primary Culture of Hepatocytes from Adult Rat Liver.” The Tohoku Journal of Experimental Medicine, vol. 152, no. 1, 1987, pp. 47–51, www.jstage.jst.go.jp/article/tjem1920/152/1/152_1_47/_article, https://doi.org/10.1620/tjem.152.47. Haque, Haroon, et al. “Effectiveness of Sodium Benzoate as a Freshwater Low Toxicity Antifoulant When Dispersed in Solution and Entrapped in Silicone Coatings.” Biofouling, vol. 21, no. 2, 2005, pp. 109–119, pubmed.ncbi.nlm.nih.gov/16109600/, https://doi.org/10.1080/08927010500222551. O’Connor, J. E., et al. “The Potentiation of Ammonia Toxicity by Sodium Benzoate Is Prevented by L-Carnitine.” Biochemical and Biophysical Research Communications, vol. 145, no. 2, 15 June 1987, pp. 817–824, pubmed.ncbi.nlm.nih.gov/3593373/, https://doi.org/10.1016/0006-291x(87)91038-2. Stefanidou, M., et al. “Assessing Food Additive Toxicity Using a Cell Model.” Veterinary and Human Toxicology, vol. 45, no. 2, 1 Mar. 2003, pp. 103–105, pubmed.ncbi.nlm.nih.gov/12678300/. Mpountoukas, P., et al. “Cytogenetic Study in Cultured Human Lymphocytes Treated with Three Commonly Used Preservatives.” Food and Chemical Toxicology, vol. 46, no. 7, July 2008, pp. 2390–2393, https://doi.org/10.1016/j.fct.2008.03.021. Whittaker, A., et al. “Toxic Additives in Medication for Preterm Infants.” Archives of Disease in Childhood. Fetal and Neonatal Edition, vol. 94, no. 4, 1 July 2009, pp. F236-240, pubmed.ncbi.nlm.nih.gov/19158148/, https://doi.org/10.1136/adc.2008.146035. Zosel, Amy, et al. “Severe Lactic Acidosis after an Iatrogenic Propylene Glycol Overdose.” Pharmacotherapy, vol. 30, no. 2, Feb. 2010, pp. 219–219, https://doi.org/10.1592/phco.30.2.219. Bailey, David N. “Propylene Glycol as a Vehicle for Percutaneous Absorption of Therapeutic Agents.” Journal of Analytical Toxicology, vol. 16, no. 2, 1 Mar. 1992, pp. 97–98, https://doi.org/10.1093/jat/16.2.97. Sood, Rohit, et al. “Quantitative Evaluation of the Effect of Propylene Glycol on BBB Permeability.” Journal of Magnetic Resonance Imaging: JMRI, vol. 25, no. 1, 1 Jan. 2007, pp. 39–47, pubmed.ncbi.nlm.nih.gov/17173307/, https://doi.org/10.1002/jmri.20802. Accessed 14 Dec. 2023. Den Hond, Elly, et al. “Human Exposure to Endocrine Disrupting Chemicals and Fertility: A Case–Control Study in Male Subfertility Patients.” Environment International, vol. 84, Nov. 2015, pp. 154–160, https://doi.org/10.1016/j.envint.2015.07.017. Jurewicz, Joanna, et al. “Environmental Levels of Triclosan and Male Fertility.” Environmental Science and Pollution Research, vol. 25, no. 6, 7 Dec. 2017, pp. 5484–5490, www.ncbi.nlm.nih.gov/pmc/articles/PMC5823964/, https://doi.org/10.1007/s11356-017-0866-5. Wang, Xiaoli, et al. “Triclosan Causes Spontaneous Abortion Accompanied by Decline of Estrogen Sulfotransferase Activity in Humans and Mice.” Scientific Reports, vol. 5, no. 1, 15 Dec. 2015, p. 18252, www.nature.com/articles/srep18252, https://doi.org/10.1038/srep18252. Geer, Laura A., et al. “Association of Birth Outcomes with Fetal Exposure to Parabens, Triclosan and Triclocarban in an Immigrant Population in Brooklyn, New York.” Journal of Hazardous Materials, vol. 323, no. Pt A, 5 Feb. 2017, pp. 177–183, pubmed.ncbi.nlm.nih.gov/27156397/, https://doi.org/10.1016/j.jhazmat.2016.03.028. Weatherly, Lisa M., et al. “Antimicrobial Agent Triclosan Is a Proton Ionophore Uncoupler of Mitochondria in Living Rat and Human Mast Cells and in Primary Human Keratinocytes.” Journal of Applied Toxicology, vol. 36, no. 6, 23 July 2015, pp. 777–789, https://doi.org/10.1002/jat.3209. López-Pacheco, Itzel Y., et al. “Anthropogenic Contaminants of High Concern: Existence in Water Resources and Their Adverse Effects.” Science of the Total Environment, vol. 690, Nov. 2019, pp. 1068–1088, tec.mx/sites/default/files/2019-08/1-s2.0-S0048969719331651-main%20%281%29.pdf, https://doi.org/10.1016/j.scitotenv.2019.07.052. Paul, Katie B., et al. “Developmental Triclosan Exposure Decreases Maternal and Neonatal Thyroxine in Rats.” Environmental Toxicology and Chemistry, vol. 29, no. 12, 15 Oct. 2010, pp. 2840–2844, https://doi.org/10.1002/etc.339. Huang, Wei, et al. “Lipid Metabolism Disorders Contribute to Hepatotoxicity of Triclosan in Mice.” Journal of Hazardous Materials, vol. 384, 15 Feb. 2020, p. 121310, www.sciencedirect.com/science/article/abs/pii/S0304389419312646, https://doi.org/10.1016/j.jhazmat.2019.121310. Rodríguez, Pablo E. A., and Mónica S. Sanchez. “Maternal Exposure to Triclosan Impairs Thyroid Homeostasis and Female Pubertal Development in Wistar Rat Offspring.” Journal of Toxicology and Environmental Health, Part A, vol. 73, no. 24, 29 Oct. 2010, pp. 1678–1688, https://doi.org/10.1080/15287394.2010.516241. Tobar, Steven, et al. “Triclosan Promotes Epicutaneous Sensitization to Peanut in Mice.” Clinical and Translational Allergy, vol. 6, no. 1, 5 Apr. 2016, https://doi.org/10.1186/s13601-016-0102-2. Park, Bo Kyung, et al. “Effects of Triclosan on Neural Stem Cell Viability and Survival.” Biomolecules & Therapeutics, vol. 24, no. 1, 1 Jan. 2016, pp. 99–107, www.biomolther.org/journal/view.html?volume=24&number=1&spage=99&year=2016, https://doi.org/10.4062/biomolther.2015.164. Pollock, Tyler, et al. “Triclosan Exacerbates the Presence of 14C-Bisphenol a in Tissues of Female and Male Mice.” Toxicology and Applied Pharmacology, vol. 278, no. 2, 15 July 2014, pp. 116–123, www.sciencedirect.com/science/article/pii/S0041008X14001574, https://doi.org/10.1016/j.taap.2014.04.017. Shim, Juyoung, et al. “Triclosan Is a Mitochondrial Uncoupler in Live Zebrafish.” Journal of Applied Toxicology, vol. 36, no. 12, 28 Mar. 2016, pp. 1662–1667, https://doi.org/10.1002/jat.3311. Lin, Dasong, et al. “Potential Biochemical and Genetic Toxicity of Triclosan as an Emerging Pollutant on Earthworms (Eisenia Fetida).” Chemosphere, vol. 81, no. 10, Nov. 2010, pp. 1328–1333, https://doi.org/10.1016/j.chemosphere.2010.08.027. Stoker, Tammy E., et al. “Triclosan Exposure Modulates Estrogen-Dependent Responses in the Female Wistar Rat.” Toxicological Sciences, vol. 117, no. 1, 1 Sept. 2010, pp. 45–53, academic.oup.com/toxsci/article-abstract/117/1/45/1682020?redirectedFrom=fulltext, https://doi.org/10.1093/toxsci/kfq180. Pearce, Elizabeth N., and Lewis E. Braverman. “Environmental Pollutants and the Thyroid.” Best Practice & Research Clinical Endocrinology & Metabolism, vol. 23, no. 6, Dec. 2009, pp. 801–813, https://doi.org/10.1016/j.beem.2009.06.003. Jm, Braun. “Early-Life Exposure to EDCs: Role in Childhood Obesity and Neurodevelopment.” Nature Reviews. Endocrinology, 1 Mar. 2017, pubmed.ncbi.nlm.nih.gov/27857130/. Zeng, Liudan, et al. “LINE-1 Gene Hypomethylation and P16 Gene Hypermethylation in HepG2 Cells Induced by Low-Dose and Long-Term Triclosan Exposure: The Role of Hydroxyl Group.” Toxicology in Vitro: An International Journal Published in Association with BIBRA, vol. 34, 1 Aug. 2016, pp. 35–44, pubmed.ncbi.nlm.nih.gov/26970259/, https://doi.org/10.1016/j.tiv.2016.03.002. Wang, Cai-Feng, and Ying Tian. “Reproductive Endocrine-Disrupting Effects of Triclosan: Population Exposure, Present Evidence and Potential Mechanisms.” Environmental Pollution, vol. 206, Nov. 2015, pp. 195–201, https://doi.org/10.1016/j.envpol.2015.07.001. Ginsberg, Gary L., and Sophie J. Balk. “Consumer Products as Sources of Chemical Exposures to Children.” Current Opinion in Pediatrics, vol. 28, no. 2, Apr. 2016, pp. 235–242, https://doi.org/10.1097/mop.0000000000000329. Buth, Jeffrey M., et al. “Dioxin Photoproducts of Triclosan and Its Chlorinated Derivatives in Sediment Cores.” Environmental Science & Technology, vol. 44, no. 12, 15 June 2010, pp. 4545–4551, sludgenews.org/resources/documents/Buth_Dioxin-Triclosan.pdf, https://doi.org/10.1021/es1001105. Christopher, M. M., et al. “Propylene Glycol Ingestion Causes D-Lactic Acidosis.” Laboratory Investigation; a Journal of Technical Methods and Pathology, vol. 62, no. 1, 1 Jan. 1990, pp. 114–118, pubmed.ncbi.nlm.nih.gov/2296157/. Yang, Qi, et al. “Analysis of Pre-Existing IgG and IgM Antibodies against Polyethylene Glycol (PEG) in the General Population.” Analytical Chemistry, vol. 88, no. 23, 16 Nov. 2016, pp. 11804–11812, https://doi.org/10.1021/acs.analchem.6b03437. The best organic diapers are not only safe for the environment but also safe for your baby. But, it is challenging to pick the best diaper brand when every other brand touts their diaper as the safest. Today, there are two sustainable diaper options - cloth diapers and disposable diapers. These two diapers help in two ways; reduce the buildup of diapers in landfills and protect your baby from the harsh chemicals, such as chlorine, in conventional diapers. With the organic diapers, your baby will not suffer the chemicals' effects, and the environment will thank you for it. Cloth diapers are baby-friendly and environment-friendly, but although they might be the best natural diapers, they are not mum-friendly, which is why organic diapers rock. When shopping for diapers, only go for a chemical-free diaper (watch out for chemicals such as parabens, phthalates, and chlorine), synthetic fragrance-free, and free of dyes. Again, ensure that all the materials used to make the diaper are natural or organic and are biodegradable. Harmful chemicals in diapersBesides making the environment unsightly, these disposable diapers contain chemical ingredients that could harm the environment, animals, and human beings. According to the Real Diaper Association, some components include polyethylene, petroleum, gelling material, perfume, and polypropylene. They also have non-renewable petroleum products. Some of the disposable diapers contain chemicals that might lead to the release of dioxin into the environment. Dioxin is a toxin linked to cancer and health concerns in fetuses. Health effects to Babies
referencesDavidson, Laurel. “The Only Way to Change Diapers Is One Baby at a Time. Real Diaper Association.” Real Diaper Association, 14 May 2015, realdiapers.org/diaper-facts.
World Health Organization. “Dioxins and Their Effects on Human Health.” Who.int, World Health Organization, 4 Oct. 2016, www.who.int/news-room/fact-sheets/detail/dioxins-and-their-effects-on-human-health. Manikkam, Mohan, et al. “Dioxin (TCDD) Induces Epigenetic Transgenerational Inheritance of Adult Onset Disease and Sperm Epimutations.” PLoS ONE, vol. 7, no. 9, 26 Sept. 2012, p. e46249, https://doi.org/10.1371/journal.pone.0046249. Accessed 12 Mar. 2019. “Chlorine “Allergy.”” ACAAI Public Website, 15 Jan. 2015, acaai.org/allergies/types/allergy-myths/chlorine-allergy. Steinemann, Anne. “Fragranced Consumer Products: Exposures and Effects from Emissions.” Air Quality, Atmosphere & Health, vol. 9, no. 8, 20 Oct. 2016, pp. 861–866, https://doi.org/10.1007/s11869-016-0442-z. Spencer, P., et al. “Neurotoxic Fragrance Produces Ceroid and Myelin Disease.” Science, vol. 204, no. 4393, 11 May 1979, pp. 633–635, https://doi.org/10.1126/science.432669. Accessed 13 Nov. 2019. Kazemi, Zahra, et al. “Evaluation of Pollutants in Perfumes, Colognes and Health Effects on the Consumer: A Systematic Review.” Journal of Environmental Health Science and Engineering, vol. 20, no. 1, 3 Feb. 2022, pp. 589–598, https://doi.org/10.1007/s40201-021-00783-x. |
This portal contains research, news, information, observations, and ideas at the level of self in an effort to address lifestyle applications.
Archives
June 2024
Categories
All
|





 RSS Feed
RSS Feed

